-
Peste des petits ruminants (PPR), also known as "Goat Plague" is highly contagious and one of the most economically important World Organization for Animal Health (OIE) notifiable diseases of sheep and goats. The causative agent, PPR virus (PPRV) is genetically grouped into four lineages (Ⅰ, Ⅱ, Ⅲ, and Ⅳ) on the basis of partial fusion (F) protein gene sequence analysis, of which lineage Ⅳis restricted to Asia and the other lineages are prevalent in Africa [8, 19]. The disease is enzootic in the
Middle East, the Arabian Peninsula and parts of Africa and Asia. Despite strict control measures including statutory regulations along with availability of various diagnostics and live attenuated PPR vaccines [5, 9, 22], the infection still remains a constant threat to livestock.
PPR is considered as one of the major constraints in improving the productivity of small ruminants in India since its first report in Tamil Nadu during 1987 [23]. In India, vaccination is the most effective means of control and the current strategy involves vaccinating small ruminants (sheep and goats) by using attenuated lineage ⅣPPR vaccines [22] along with sero-monitoring/surveillance using monoclonal antibody based PPR competitive ELISA (c-ELISA) [25]. Currently available PPR vaccines [22] including thermo-adapted [5] and deuterated [18] PPR vaccines may suffice to protect against the circulating PPRVs in India. The former vaccine provides long-term protective immunity in goats [15] and has undergone extensive field trials in sheep and goats, apart from efficacy, thermo-stability, immunogenicity and pathogenicity [11, 16, 17, 20].
PPR is enzootic in India and vaccination has routinely been employed since 2002 [20]. The age at which offspring can effectively be immunized is proportional to the amount of protective antibody they received from their dams. Protective immunity can be produced through vaccination, when the maternal antibodies recede to a low level in kids, whereas high levels of maternal antibodies would block the effectiveness of the vaccine. There is a window of susceptibility period from several days to weeks in which the maternal antibodies are too low to provide protection against the disease, but too high to allow a vaccine to work. This is the period where a young animal can still contract the disease despite being vaccinated. The efficacy of the African lineage vaccine strain against PPR and its colostral immunity had been previously demonstrated [2].
Therefore, the present study was conceived to investigate the decay of maternal PPRV antibody in kids born to goats vaccinated with Asian lineage vaccine and the efficacy of the passive immunity against PPRV to determine the optimum period (at which maternal antibodies probably would no longer interfere with vaccination of kids) for vaccination in goats.
HTML
-
Goats of either sex (aged 6 to 12 months) were procured from sub-Himalayan regions, Uttarakhand, India and maintained at the quarantine animal facility of the institute. The animals were de-wormed against gastrointestinal helminthes using albendazole and rectal temperature was recorded for identifying any infection prior to experiment. The Animal Ethics Committee norms were followed for the experiment as a part of routine PPR vaccine testing being carried out in goats. All the healthy animals were housed separately with adequate containment and sero-screened for PPRV antibodies by PPR c-ELISA and serum neutralization test (SNT) [13, 25]. Eight sero-negative goats {two males (buck) and six females (doe) including one pregnant doe} were used for vaccination with one field dose (103TCID50) of live attenuated thermo-adapted PPRV Sungri strain [5]. Two recovered PPR virulent virus challenged female goats (as infected animals) and two healthy unvaccinated female goats (as controls) were also included in the study. The kids born to unvaccinated healthy goats aged between 4 to 6 months were tested in PPR c-ELISA and sero-negative kids were used as control in the challenge study.
-
All the vaccinated goats were observed daily and rectal temperature was recorded for a period of 21 days post-vaccination (DPV). The goats were sampled (blood collected through jugular vein puncture) periodically at different intervals, initially weekly, then monthly and later quarterly as per an earlier study [15] up to one year (0, 7, 14, 21, 45, 56, 90, 120, 225 and 310 DPV). To monitor maternal antibodies, sampling was done in the kids born to either immunized or infected or unvaccinated dams up to six months of age. As synchronized breeding was not done, kids were included in the study as and when they were born. All kids born during the study period were sampled.
-
The serum samples were tested for PPRV haemagglutinin (H) antibodies in terms of percentage inhibition (PI) as per Singh et al. [25]. Serum samples showing PI > 40 were considered positive for the presence of PPRV antibodies.
-
For the detection of neutralizing antibodies to PPRV and to determine the SN titer, a microassay was carried out in Vero cells as described earlier [6, 13]. Briefly, serum samples were diluted two-fold serially in a 96-well tissue culture plate and incubated with 100 TCID50 of PPR vaccine virus sungri-96 strain in a 5% CO2 humidified incubator at 37℃ for 2 h. Vero cell suspension (105cells in 100 µL) was added to each well and the plates were further incubated at 37℃ in a humidified CO2incubator for 7-8 days under regular observation until the cytopathic effects (CPE) were evident with change of maintenance media on alternative days. The appropriate cell, virus and serum controls were included for each plate. After visual observation of PPRV-specific CPE on the sixth day, final readings of the microtitre plates were re-confirmed by the cell-ELISA method [3, 21] to detect the presence of virus as described earlier. The SN titers were calculated as the reciprocal of the highest dilution of serum that shows no CPE in 50% of wells. The protective levels of antibodies in kids' serum samples were analyzed and the SN titers of 1:8 and above were considered as protective [13].
-
One of the kids born to a vaccinated dam having an SN titer of 1:8 was actively challenged at 4 months of age for assessing the protective efficacy of the passive immunity. Similarly, another kid at 4 months of age having a passive 1:8 SN titer was immunized with one field dose vaccine and assessed for PPR protective antibody response on 21dpv followed by a virulent virus challenge in order to determine the interference of the passive immunity with vaccination ie., the protective response of vaccine virus.
In the challenge study, these two kids along with other two sero-negative control kids were experimentally infected with 2 mL of goat-adapted highly virulent challenge PPRV (Izatnagar 94 isolate) subcutaneously at two different sites in the form of 10% splenic suspension needle passaged virus in goats as described previously [16] and observed for the development of any clinical signs specific to PPR. The amount of nucleocapsid (N) protein in 1 mL of suspension was estimated by anti-PPRV N protein MAb-based sandwich-ELISA (s-ELISA) [24], which is equivalent to the amount of N protein present in 1 mL of PPR vaccine (titer of 105.5 TCID50/mL).
From this experiment, the nasal, ocular and oral swabs were collected periodically {from day 1 to 20 days post challenge (DPC)} from all the challenged goats during the course of infection. The collected samples were tested for detection of either PPRV antigen (swab materials were directly squeezed or extracted with 0.5 to 1 mL phosphate buffered saline-PBS) or nucleic acid sequences (viral RNA was extracted from the swab materials by using QIAamp Viral RNA kit) by s-ELISA [24] and Matrix (M) gene based RT-PCR assay [4], respectively.
Animals
Sampling
Competitive ELISA
Serum Neutralization Test (SNT)
Challenge study
-
All vaccinated animals which were maintained for a study period of one year (so far tested) developed protective antibody levels two weeks after vaccination, with 61 to 96 percentage inhibition (PI) of PPRV H antibodies when tested by PPR c-ELISA (Fig. 1). Control unvaccinated goats did not show any positive PPRV-specific antibodies threshold (had PI value of 35 to 35.9 in c-ELISA). The vaccinated, infected and unvaccinated goats became pregnant. A total of four kids were born to vaccinated goats at different intervals over a period of time.
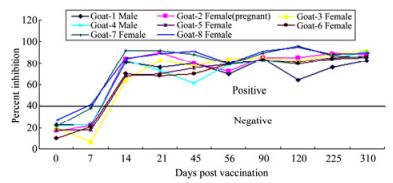
Figure 1. Sero-monitoring of PPR vaccinated goats by using PPR competitive ELISA. Solid line shows negative/positive cut-off. Samples tested positive for PPRV H antibodies after 21st days post-vaccination (DPV) with PI value ranging from 61 to 96.
After 3–4 weeks of vaccination, the first kid was born to vaccinated pregnant doe number 2. The range of intervals between dam vaccination and kid delivery are shown in Table 1 with PI value of PPRV H antibodies in the dams near to the time of parturition. Maternal antibodies in kids born to vaccinated goats were detectable up to 6 months of age but decline in antibody titers was observed at slow rate from the second month and rapid rate from the third month and it finally reached below the protective or negligible level at the fourth month with no significant differences in both sexes. Similarly, two kids born from the infected dams had a similar pattern of maternal antibody decay with a protective titer at the age of 5-6 months. However, the three kids born from the unvaccinated dams did not show any PPRV-specific passive antibodies. Maternal antibody decay in kids born from immunized or exposed/infected dams is presented in Table 2.

Table 1. Antibody titers in vaccinated female goats at the time of kidding

Table 2. Pattern of maternal antibody decay in kids born to vaccinated or infected dams
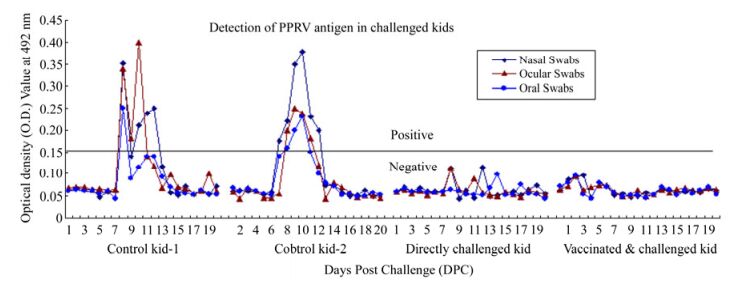
One of the kids having an SN titer of 1:8 was vaccinated, which had significant PPRV-specific protective antibody response (PI = 76 and SN titer > 1:16) when tested on 21 dpv and was completely protected from a virulent virus challenge. Similarly, a directly challenged kid having an SN titer of 1:8 was completely protected from PPR. Furthermore, PPRV antigen/ nucleic acid was not detectable by s-ELISA and RT-PCR assay in the natural secretions of post challenged kids, whereas the experimentally infected control kids showed pyrexia (105.6 ℉ or 40.9 ℃) and naso-ocular discharges from day 4 post infection (DPI), and the collected samples (nasal, ocular and oral swab) were found positive for PPRV antigen /nucleic acid sequences. Using RT-PCR, the detection of virus nucleic acids in these samples as early as from 5th to 15th DPI was observed. The same samples, when tested by s-ELISA, detected the virus antigen in clinical swabs at 7–12th DPI. The details of results of virus identification in the experimental study by s-ELISA and RT-PCR are shown in Table 3 and depicted in Fig. 2.

Table 3. Details of results of virus identification in experimental challenge study
-
Maternal vaccination is necessary to provide protective passive immunity to the neonate against infections, which has prompted widespread interest and has been successfully employed in the animal health industry [10]. However, a range of animal models and human studies indicate potential inhibitory effects of maternal antibody on vaccine-specific humoral responses [14]. Providing protective immunity to neonatal animals in early life is associated with numerous challenges with respect to vaccine safety and efficacy. In India, the decrease in the number of PPR outbreaks in sheep and goats in the recent years might be due to appropriate time of vaccination, the efficacy of vaccine and circulation of only a Asian lineage Ⅳvirus [5].
This study shows the efficacy of field doses of the vaccine reported earlier during long-term vaccine trials in goat [15]. As determined earlier [15, 16, 25], the PPRV H antibodies were maintained above the threshold PI value (40%) and ranged between 61 to 96, indicating strong positivity, which may be suggestive of protective humoral response (protective SN titers ≥ 1:8). The kids (as and when they were born) to these pregnant dams were assessed for the protective efficacy of the maternal antibody to determine the window of susceptibility.
The results indicated a considerably higher level of antibodies in the vaccinated goats prior to kidding (Table 1). In general, timing of vaccination will be dependent on the titers in the dams, and thus may vary depending on the vaccine used. Young animals born to immune dams might still have protective antibody levels at the age of 5-6 months. In this study, the kids born to the infected animals have protective SN titers at the age of 5 to 6 months, which may be due to the higher titers of the PPRV antibodies in the infected dams. In our earlier studies on the kinetics of PPRV antibodies in the recovered challenged / infected goats we showed that they had PI range of 90-95, when the serum samples were tested by PPR c-ELISA. Similarly, in this study, the infected dams also had PI value ranging from 89 to 92 near to the time of parturition (Table 1). Furthermore, the kid born from dam (doe number 2) had protective SN titer at the age of 5 months, which may also be due the time of PPR vaccination, as this doe was vaccinated during pregnancy. However, the kid born to dam (doe number 3) had lost protective SN tire at the age of 4 months which may be due to idiosyncrasy of the animal. This study in general indicated that the kids should be vaccinated after 4 months of age to avoid the window of susceptibility.
In general, the passive serum antibody titers revealed mounting of a protective humoral response in these kids at earlier months. It is important to consider the slow decay of (two fold) antibody in the first two months compared to the rapid decay in the later two months. In the first two to three weeks of life, there was an increased PI value, which could be due to transfer of antibodies through colostrums as both dam and its kids, were let free for enough colostrums feeding. This is in concurrence with the earlier report of passively acquired bovine viral diarrhea virus antibodies that prevented clinical disease in inoculated colostrum fed calves at 2 to 5 weeks of life; calves were still protected from clinical disease after passive serum antibody titers had decayed to non-detectable levels [12]. Similarly, Awa et al. [2] reported that less variation in the decay of PPR antibody in the early months of the offspring will not alter the antibody titer in the dam. The results for the kids are in agreement with those reported earlier, for the kids aged 3 to 4 [1] and 4 to 5 months [7]. In this study, the decay of PPR maternal antibody in kids correlates with the earlier report [7] indicating that the duration of maternal immunity to PPRV ranges from 4 to 5 months with respect to the PPR vaccine (Nigerian lineage vaccine). Further, these findings have implications for evaluating vaccine efficacy and immune status of the animals as reported earlier [12]. Although lineages reflect the sequence diversity of a small portion of the F gene, the protective titer may remain the same for either PPR vaccine.
The SN titers of ≥1:8 were considered as protective in case of PPR [13, 16]. As these kids were born at different points of time, only two kids (one each from vaccinated and infected animals) were suitable to challenge with SN titer of 1: 8, and were included in the study at ~4 month post partum period (one kid for vaccination and another kid for direct challenge). By this time, the vaccinated animals should cover a minimum period of 21 days, so as to subject them for challenge simultaneously along with two healthy kids (aged between 4 to 6 months old). The challenge experiment was conducted simultaneously in order to avoid experimental variations even though only one animal in each group was statistically insignificant. Therefore, the further development of the antibody titers in the vaccinated kid after 21st DPV has not been investigated. Moreover, it is well known that morbillivirus-specific humoral antibodies with protective titers provide protection in general. In our earlier study, PPR vaccine provides a protective immunity as early as 21 dpv and, which has been maintained over a period of
$6{}^{1}\!\!\diagup\!\!{}_{2}\;$ years (so far studied) [15]. In this experiment, the virus identification or detection in the infected control kids by diagnostic assays indicates an active infection by challenge experiment as reported earlier [4]. Furthermore, the detection of virus nucleic acid and PPRV antigen in these samples at the described intervals was also concurrent with the earlier reports [4, 24].Even though passively acquired antibodies have been studied with respect to PPR vaccines, other immunological mechanisms may be significant in providing maternally derived immunity in sheep and goats. From this preliminary attempt, the maternal antibodies in kids born to either vaccinated dams with PPR lineage Ⅳvaccine or PPRV infected dams were detectable up to 5-6 months with a decline in the protective level of antibodies from the fourth month onwards. Therefore, PPR vaccination may be recommended after 4 months in kids born to immunized or exposed dams, which is a suitable period to evade the window of susceptibility to protect the young kids as well as to eliminate PPR infection in susceptible populations. Further, systematic study on the correlation between maternal/offspring PPRV antibody and protective efficacy of the material antibody with other immunological parameters in large population of animals by synchronized breeding is warranted.













 DownLoad:
DownLoad: