-
Duck virus hepatitis (DVH) is an acute and fatal disease of ducklings, characterized (primarily) by liver necrosis, hemorrhage and high mortality. In 1949, DVH was first described in Long Island [11]. The disease is caused by three different duck hepatitis virus (DHV) types 1, 2 and 3 and no antigenic relationships have been found between them [5, 24, 26]. Among the three serotypes of DHV, the most common and most virulent serotype worldwide is DHV-1 [1, 11].
DHV-1 was originally classified as an enterovirus based on the observed morphology and physicochemical properties of the virion [25]. However, based on phylogenetic analysis, it was recently found that DHV-1 was more closely related to members of the genus Parechovirus than to other picornaviruses [9]. In the Virus Taxonomy ninth Report of the International Committee on Taxonomy of Viruses (ICTV), DHV-1 was classified as a member of a novel genus Avihepatovirus in the family Picornaviridae and renamed as duck hepatitis A virus (DHAV) (ICTV, 2009). Based on phylogenetic analyses and neutralization tests, DHAVs has been classified into three serotypes: DHAV-1 (the classical serotype 1) [2, 9, 22], DHAV-2 (a serotype recently isolated in Taiwan) [23] and DHAV-3 (the recently described serotype isolated in South Korea and China) [3, 10]. There is no cross-neutralization between DHAV-1 and DHAV-2 [23] and limited cross-neutralization between DHAV-1 and DHAV-3 [10].
As the most virulent serotype, DHAV-1 usually affects ducklings under 3 weeks of age and is distributed worldwide. In China, outbreaks of DVH have occurred in many ducklings that were already vaccinated with the traditional DHAV-1 attenuated vaccine. Thus a major concern is whether the variation of virulence or variation in the external capsid proteins of the pandemic DHAV-1 isolates caused the immune failure. In this study, the ELD50s and LD50s of nine DHAV-1 strains isolated from Shandong province of China were tested in duck embryos and ducklings, hyperimmune sera against the isolates were produced, cross neutralization assays were done and the VP1 genes were amplified, sequenced and analyzed.
HTML
-
Nine DHAV-1 strains (named LQ43, LQ44, LQ45, LQ54, LQ57, WS12, WS45, LY01, SG01) were isolated from Cherry Valley ducks in Shandong province China from January 2007 to November 2008. The DHAV-1 infections were found in one-week-old ducklings in the farms. The mortality of duck flocks was 20%–80%, and the sick ducklings died quickly with typical hemorrhagic hepatitis.
-
The fifth generation duck embryo allantoic liquids of the 9 DHAV-1 isolates were diluted by sterile 0.9% NaCl to 10-2, 10-3, 10-4, 10-5, 10-6, 10-7 and 10-8, respectively. Seven different gradient allantoic liquids of every isolate were inoculated into 12-day-old non-immune duck embryonated eggs through the allantoic route, 0.2 mL per egg and ten eggs per gradient. At the same time ten embryos were inoculated with sterile 0.9% NaCl (0.2 mL per egg) as control. Embryonic duck eggs were incubated at 37 ℃ for 96 h. The numbers of embryos that died within 24-96 h after inoculation were recorded and the ELD50s were determined by the Reed and Muench method [19].
-
For each DHAV-1 isolate, 50 7-day-old healthy ducklings without the neutralization antibody against DHAV-1 were divided into five groups, and diluted fifth generation duck embryo allantoic liquids of five gradients (10-3, 10-4, 10-5, 10-6 and 10-7) were inoculated by intramuscular injection to duckling legs, 0.2 mL per duckling and ten ducklings per gradient. Another ten ducklings were injected with sterile 0.9% NaCl (0.2 mL per duckling) as negative control. The numbers of ducklings that died within 96 h post-inoculation were recorded for each gradient and the LD50s of the 9 viruses were calculated using the Reed-Muench method [19].
-
Standard hyperimmune serum against DHAV-1 was purchased from the China Institute of Veterinary Drug Control (Beijing, China). Hyperimmune sera against the 9 DHAV-1 isolates were produced in 4-week-old SPF chickens maintained at the College of Veterinary Medicine, Shandong Agricultural University, China. The birds were immunized at a dose of 107.0 ELD50 per bird, once orally and three times intramuscularly at weekly intervals. The hyperimmune serum was inactivated at 56 ℃ for 30 min. Cross-neutralization assays were done with hyperimmune sera against the 9 DHAV-1 isolates and the standard hyperimmune serum. Briefly, the 9 isolates were each 10-fold serial diluted and incubated with equal volumes of hyperimmune serum at 37 ℃ for 60 min. The serum-virus mixture was then inoculated into 12-day-old nonimmune embryonated duck eggs by the allantoic route. At the same time, only sera and only viruses were inoculated as control. Inoculated embryonated duck eggs were incubated at 37 ℃ and were observed for up to four days.
-
The viral RNAs were extracted from allantoic liquids of dead duck embryos, which had been inoculated with the 9 respective DHAV-1 isolates, with a QIAamp® Viral RNA Mini Kit (Qiagen, Valencia, CA) according to the manufac turer's instructions.
-
RT-PCR was performed for amplification of the VP1 gene of the 9 DHAV-1 isolates with primer P1(5'-GTCAATCGACTC ACT GTG-3') and primer P2(5'-GCTCTTGCCACTTCCTGA T-3'). RT-PCR was performed utilizing a one step RNA PCR kit (TaKaRa, China). The RT-PCR condition was 50 ℃ for 30 min, 94 ℃ for 2 min, and then 30 cycles of 94 ℃ for 30 s, 50 ℃ for 30 s, 72 ℃ for 1 min, and with a final step of 72 ℃ for 5 min. PCR products were purified using a gel purification kit (Invitrogen, USA), and the purified PCR-products were TA-cloned into the pMD18-T vector (TaKaRa, China) following the manufacturer's protocol, and then were sent to a commercial service for sequencing (Shanghai Sangon Biological Engineering Technology & Service Co., Ltd).
-
The VP1 gene sequences of other DHAV-1 isolates were retrieved from GenBank (Table 1). Sequences were aligned by the ClustalW method within the DNAstar software package (DNAStar Inc. Madison, WI) programs. Phylogenetic analysis was done by the Neighbor-Joining method (Tamura-Nei distances) using MEGA 5.0 [21] with 1000 bootstrap replicates.

Table 1. The accession numbers of DHAV-1s used for comparison in this study
Virus isolates
ELD50s test
LD50s test
Cross-neutralization tests
Isolation and purification of DHAV-1 RNA
Reverse transcription-polymerase chain reaction (RT-PCR) and sequencing of VP1 genes of DHAV-1 isolates
Sequence analysis
-
Embryonic duck eggs began to die from 32 h post-inoculation by the 9 DHAV-1 isolates, and all of the dead embryos presented the typical lesions associated with DHAV infection, while the negative controls were all surviving. The ELD50s of embryonic duck eggs of the 9 DHAV-1 isolates were between 1.9 × 106/mL to 1.44 × 107/mL using the Reed-Muench method and according to the recorded numbers of duck embryos that died within 24-96 h post-inoculation.
-
Ducklings began to display clinical signs including lethargy, fastidium, weakness, lateral recumbency, epileptic seizures, and opisthotonos 24 h post-inoculation with allantoic liquid of the respective 9 DHAV-1 isolates. The sick ducklings died quickly after presenting clinical signs. Gross lesions tended to be restricted to the livers, which were swollen, fragile, and presented multiple punctate hemorrhages and ecchymosis. Under microhistological examination, the DHAV-1 isolates were shown to induce pathological changes including liver cellular necrosis and hemorrhage in infected ducklings' liver, compared to healthy ducklings' liver tissue slices. By calculating the recorded numbers of ducklings that died within 96 h post-inoculation, the LD50s of the 9 DHAV-1 isolates were 2.39 × 105/mL to 6.15 × 106/mL.
-
The 10-3 titer of the 9 DHAV-1 isolates were completely neutralized by the standard serum and the hyperimmune sera against the 9 DHAV-1 isolates, respectively. The negative control group with virus only all died within 24-96 hour, whereas the group with serum only all survived.
-
The VP1 genes of the 9 DHAV-1 isolates were successfully amplified by RT-PCR. Further analysis indicated that all these VP1 genes were 714 bp long and coded 238 amino acids. Amongst the 26 different DHAV-1 strains, the VP1 genes showed 89.8%-100% nucleotide and 92.0%-100% amino acid sequence identity. The nine strains determined in this paper shared 90.9%-99.7% similarity at the nucleotide level and 94.1%-99.6% at amino acid level, and shared 89.8%-99.7% nucleotide similarity and 92.4%-99.6% amino acid similarity with other DHAV-1 strains (Table 2). There were three hypervariable regions (HVR) at the C-terminal of VP1 amongst the different DHAV-1 strains (Fig. 1). Only four strains (LQ43, LQ44, LQ54 and WS12) determined in this study showed three consistent differences from the other strains (G158K159S160-L158S159V160) in the HVR1, while the strains with different virulence showed more variation in the HVR2 and HVR3 regions.

Table 2. Pair-wise comparisons between the nucleotide (upper right) and amino acid (lower left) sequences of the VP1 gene of DHAV-1 strains
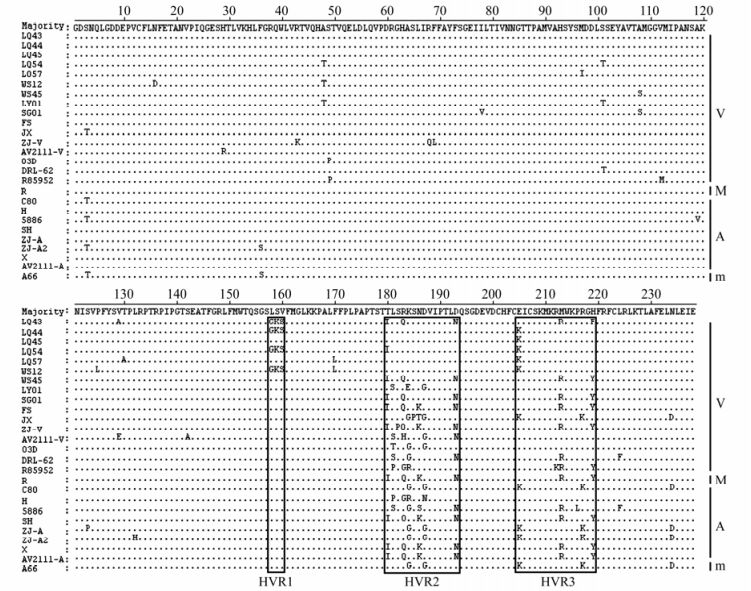
Figure 1. Amino acid sequence alignment of the VP1 protein of DHAV-1 isolates. The first nine lines are the isolates from Shandong province of China determined in this study. Residues are shown only where they differ from the top line (Majority). The hypervariable regions (HVRs) at the C-terminus are shown in box. The right letters: V, virulent strain; M, moderate virulent strain; A, attenuated vaccine strain; m, mild strain.
The phylogenetic tree of the VP1 gene nucleotide sequences showed that all the 26 DHAV-1 strains were clustered into four major genogroups (GⅠ, GⅡ, GⅢ and GⅣ, Fig. 2A). These genogroups contained DHAV-1 strains showed significant correlation with their geographical origin. GⅠ contained 8 strains isolated from China, GⅡ contained 3 American strains (DRL-62, R85952 and 5886) and 1 HK attenuated vaccine strain (H), GⅢ contained 7 Chinese isolates, and GⅣ contained 1 Taiwan isolates (03D) and other 5 Chinese virulent isolates.

Figure 2. Phylogenetic (neighbor-joining) tree of DHAV-1 VP1 gene nucleotide sequences (A) and amino acid sequences (B). The values at the forks indicate the percentage of trees in which this grouping occurred after bootstrapping the data (1000 replicates; shown only when > 50%). The scale bar shows the number of substitutions per base. The isolates in this study were marked with asterisks (*).
The 26 DHAV-1 strains were clustered into two major genogroups (GI and GⅡ, Fig. 2B) in phylogenetic tree of VP1 gene amino acid sequences. GI contained 15 different virulent isolates, and can be further divided into 3 subgroups (S1 to S3). S1 included 7 Chinese virulent isolates (JX, LY01, LQ44, LQ45, LQ54, LQ57 and WS12), 1 Taiwan virulent isolates (03D), 3 Chinese attenuated vaccine strains (C80, ZJ-A and ZJ-A2), and 1 Chinese mild strain (A66), S2 contained 1 American virulent isolate (R85952) and 1 HK attenuated vaccine strain (H), while S3 contained a single Chinese virulent isolate AV2111-A. GⅡ included 11 different virulent isolates, and can be further divided into 2 subgroups (S1 and S2). S1 contained 2 American virulent isolates (DRL-62 and 5886), while S2 included 6 Chinese virulent isolates (LQ43, WS45, SG01, FS, ZJ-V and AV2111-V), 1 Chinese moderately virulent strain (R), and 2 Chinese attenuated vaccine strains (SH and X).
ELD50s of embryonic duck eggs
Animal experiments and LD50s
Cross-neutralization
Sequence analysis of VP1 gene
-
VP1 is the most external capsid protein in Picornaviruses and containing the primary neutralization epitope [20]. This has been shown in other members of Picornaviridae such as FMDV and Encephalomyocarditis virus. Within the same species of picornaviruses, the most distinctive divergence is usually located in VP1 [7, 16]. The VP1 nucleotide and amino acid pairwise percent identity scores can be used to determine the serotype identity of enterovirus isolates. The criteria for the same serotypes are the VP1 nucleotide identity ≥75% and/or amino acid identity ≥88% [16-18]. In this paper, 9 DHAV-1 strains isolated from Shandong province of China were virulent strains with an ELD50s of embryonic duck eggs between 1.9 × 106/mL to 1.44 × 107/mL and the LD50s of duckling between 2.39 × 105/mL to 6.15 × 106/mL. By cross-neutralization tests, the 9 DHAV-1 isolates were completely neutralized by the standard serum and the hyperimmune sera against the 9 DHAV-1 isolates, respectively. The VP1 genes of the 26 DHAV-1 strains showed 89.8%-100% nucleotide and 92.0%-100% amino acid sequence identity (Table 2). Phylogenetic analyses and cross-neutralization tests showed that all the 26 DHAV-1 isolates with different virulence were the same serotype.
In the genome of DHAV, mutations of nucleotide and amino acid are mainly contained in VP1, so the VP1 was presumed might be the major virulent determinant of DHAV-1 [8]. The C-terminal region of VP1 is most highly diverse between different DHAV-1 strains, and some of amino acid mutations might lead to isolate attenuation [14]. The nucleotide and amino acid identities in VP1 region within the 16 virulent strains were 90.5%-99.7% and 92.4%-99.6%, those within the 8 attenuated vaccine strains were 92.4%-100% and 93.7%-100%, whereas those between members of any of virulent strains, moderate virulent strain, attenuated vaccine strains and mild strain were 89.8%-100% and 92.0%-100% (Table 2). Phylogenetic analyses showed the genogroups containing the DHAV-1 strains showed significant correlation with the geographical origin, but without virulence. There were three HVRs at the C-terminus (158-160, 180-193 and 205-219) and other variable points in VP1 protein, but which didn't cause change in the virulence of DHAV-1 isolates.
In the clinical cases, the ducklings were often co-infected with different DHVs. Mixed DHAV-1 and DHAV-2 infections are common in Taiwan [22]. Currently, DHAV-1, DHAV-3 and duck astrovirus (DastV) are prevalent in the mainland of China [2, 4, 8, 13, 25, 26]. Recently, DHAV-3 was detected in geese, which showed new epidemiological characteristics of DHAV [12]. This paper reported the genetic variation of the VP1 gene of the virulent DHAV-1 isolates in Shandong province of China, which will be helpful for understanding of the molecular epidemiology of DHAV-1.













 DownLoad:
DownLoad: