HTML
-
Respiratory syncytial virus (RSV) is a negative-stranded RNA virus of the Paramyxoviridae family; its genome encodes seven structural proteins and four non-structural proteins. Among the structural proteins, G protein is an attachment protein and F protein is a fusion protein involved in viral pathogenicity. G protein attachment to host cell membranes facilitates F-mediated membrane fusion. F protein can bind to cell membranes independently (via binding to cellular heparin sulfate and nucleolin (Harcourt et al., 2004; Tayyari et al., 2011)) and mediate virus infection in the absence of G (Techaarpornkul et al., 2002; Collins et al., 2007). Both F and G proteins induce production of neutralizing antibodies (Bradley et al., 1990; Belshe et al., 1993).
RSV was first discovered in 1952 (Collins et al., 2007). RSV infections cause serious lower respiratory tract illnesses, including croup, cough, bronchitis, bronchiolitis, and pneumonia (Paramore et al., 2004; Mandell et al., 2005; Ruuskanen et al., 2009). Recently, RSV has been identified as the primary cause of bronchiolitis and pneumonia among infants and young children worldwide (Hall et al., 2009; Weinberg et al., 2009). The severe lower respiratory tract illness induced by RSV is commonly seen in infants from 6 weeks to 6 months old, with a peak at the age of 2–3 months (Collins and Melero, 2011). Over half of all infants in the United States are infected in their first year of life and nearly 100% are infected by the age of two. The global annual mortality of children with lower airway infections induced by RSV is approximately 160, 000, and more inclusive all-cause mortality rates related to RSV approach 600, 000 (Glezen et al., 1986; Empey et al., 2010; Nair et al., 2011). Un controlled RSV replication and persistent inflammation lead to substantial morbidity and mortality via blockage of the small airways in infants (Collins et al., 2007; Collins and Melero, 2011; Smith et al., 2012; Glenn et al., 2013). Although it is traditionally regarded as a pediatric pathogen, elderly individuals and patients with heart or lung diseases or immunodeficiency disorders are also at risk of severe lower respiratory tract diseases (Thompson et al., 2003; Collins et al., 2008; Blanco et al., 2010; Collins and Melero, 2011).
Despite the importance of RSV as a respiratory pathogen, which has been recognized for over 40 years, the development of an RSV vaccine has been challenging. A licensed vaccine, the best single healthcare solution to an infectious disease, is currently unavailable (Rudraraju et al., 2013). Recent formulations of RSV vaccine candidates have focused on subunit vaccines (e.g., purified fusion protein [PFP] and RSV F nanoparticle vaccines), subunit vaccines combined with nonspecific immune-activating adjuvants, and live attenuated vaccines (Piedra et al., 2003). Another RSV antigen, the small hydrophobic protein SH, is also a vaccine candidate (Schepens et al., 2013). Unfortunately, none of these methods is efficacious, and an effective vaccine or treatment is urgently needed. In the current meta-analysis, the effect of vaccine prophylaxis on severe respiratory syncytial virus infection was quantified to aid in the development of methods for the prevention of RSV infection.
-
In this meta-analysis, 5 subunit vaccines were included. Three RSV subunit vaccines, purified F protein 1 (PFP-1), PFP-2, and PFP-3, have been evaluated in clinical trials in elderly individuals (Falsey and Walsh, 1996; Falsey and Walsh, 1997), healthy children older than 1 year (Tristram et al., 1996; Paradiso et al., 1997; Piedra et al., 2003; Thompson et al., 2003; ), or children with cystic fibrosis or bronchopulmonary dysplasia (Groothuis et al., 1998). RSV F nanoparticle vaccine has been studied in a population of healthy adults (Glenn et al., 2015). And the sanofi-pasteur vaccine has been studied in a randomized controlled trial in a high-risk elderly population and a general population of persons ≥ 65 years, respectively (Falsey et al., 2008; Langley et al., 2009). The F protein accounts for approximately 90%–95% and 98% of the total protein contents for PFP-1 and PFP-2, and for greater than 95% instead of the attachment (G) protein, the other major surface protein, accounts for less than 2% of the total viral protein content in PFP-3 (Welliver et al., 1994; Piedra et al., 2003). The purity of RSV F particles was estimated to be ≥ 98% in an RSV F nanoparticle vaccine (Smith et al., 2012). Sanofi-pasteur vaccine contains purified RSV-A antigens F, G and M extracted from a RSV-A working seed strain virus grown on vero cells. The F and M proteins are highly conserved while the G protein is approximately 50% conserved between the RSV subtypes.
The inclusion and exclusion criteria for published studies were defined in advance. The inclusion criteria were as follows: 1) published in English; 2) study designs were randomized and included double-blind vaccine and placebo control groups; 3) control groups received a placebo or another vaccine; 4) RSV vaccine was considered; and 5) clinical trials were performed in humans. A hierarchical system was used for the exclusion criteria, in which reasons were ranked in the following order: 1) absence of placebo or no treatment arm; 2) allocation not randomized; 3) non-English language; and 4) absence of valid data (which were not publically available or the appropriate parameters were not estimated in the studies).
-
The methodology described by the Cochrane Collaboration was followed for this meta-analysis (Julian and Sally, 2000). Randomized clinical trials were sought through the PubMed, Cochrane, and EMBASE databases using search term 'RSV vaccine'. Only human studies published between Jan 1973 and Sep 2015 were considered. All studies that utilized random allocation, included a control group that did not receive the RSV vaccine, and enrolled human subjects were eligible for review. At least two articles described each vaccine type.
-
Odds ratios (ORs) with 95% confidence intervals (CI) were determined based on each of the study outcomes.
All statistical analyses were implemented in Stata 12.0 (StataCorp LP, College Station, TX, USA). A meta-analysis of incidence data was performed and pooled estimates and 95% CIs were determined using the random effects model. The meta-analysis data was not limited with respect to placebo, age, or control product.
Study selection
Data sources and search strategy
Statistical methods
-
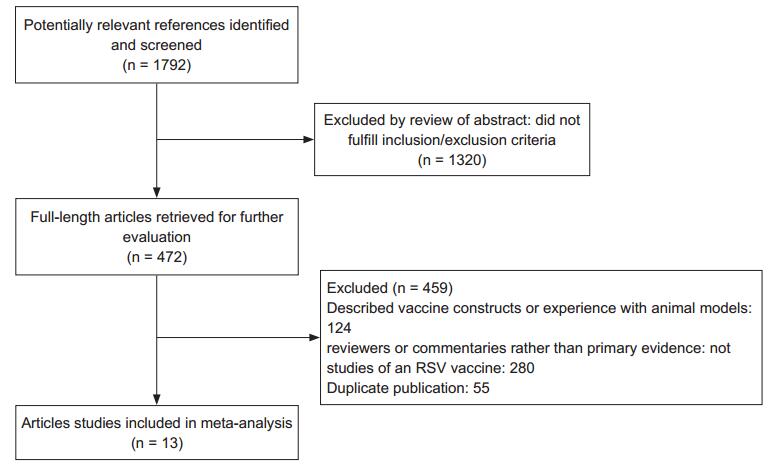
A flow diagram of the trial identification and selection procedure is shown in Figure 1. The initial search returned 1, 792 articles from the PubMed, Cochrane, and EMBASE databases. After screening article titles, 472 potentially relevant references were identified. The abstracts of these 472 articles were further reviewed and 459 full-length articles were retrieved. Thirteen articles met our inclusion criteria. Of the 11 studies evaluated the effects of vaccine prophylaxis on severe respiratory syncytial virus infection, three utilized PFP-1 (Tristram et al., 1993; Paradiso et al., 1994; Piedra et al., 1995), six utilized PFP-2 (Welliver et al., 1994; Falsey et al., 1996; Piedra et al., 1996; Groothuis et al., 1998; Piedra et al., 1998; Munoz et al., 2003), one utilized PFP-3 (Piedra et al., 2003) and one utilized an RSV F nanoparticle vaccine (Glenn et al., 2015). Meanwhile, in the four researches focused on the effect of an adjuvant, one utilized PFP-2 (Munoz et al., 2003), two utilized sanofi-pasteur vaccine (Falsey et al., 2008; Langley et al., 2009) and one utilized RSV F nanoparticle vaccine (Glenn et al., 2015), respectively.
The characteristics of the included studies are summarized in Table 1. With one exception (Piedra et al., 1996), all studies employed simple randomization. All trials were truly blind. The control group received a saline placebo in eight studies, and two trials used an influenza vaccine as a control (Piedra et al., 1995; Groothuis et al., 1998) and one trial's control was aluminum phosphate.

Table 1. Studies included in the RSV vaccine meta-analysis.
Figure 2 illustrates the effects of vaccine prophylaxis on severe RSV infection. The OR for the RSV vaccines was 0.34 (95% CI, 0.16–0.71). The data demonstrated that RSV subunit vaccines were able to significantly reduce the incidence of RSV infection, although the test of heterogeneity among studies was significant (p = 0.033). According to Tristram's research about PFP-1 (OR = 0.03, 95%CI: 0.00–0.65) and Munoz's study about PFP-2 (OR = 0.00, 95%CI: 0.00–0.07), we found that PFP vaccines are effectively to reduce the incidence of RSV infection, and their clinical value could be considered. Moreover RSV F nanoparticle vaccine appeared safe, immunogenic, and reduced RSV infections estimated by Glenn (2015) since the OR was 0.44 (95%CI: 0.21–0.93).
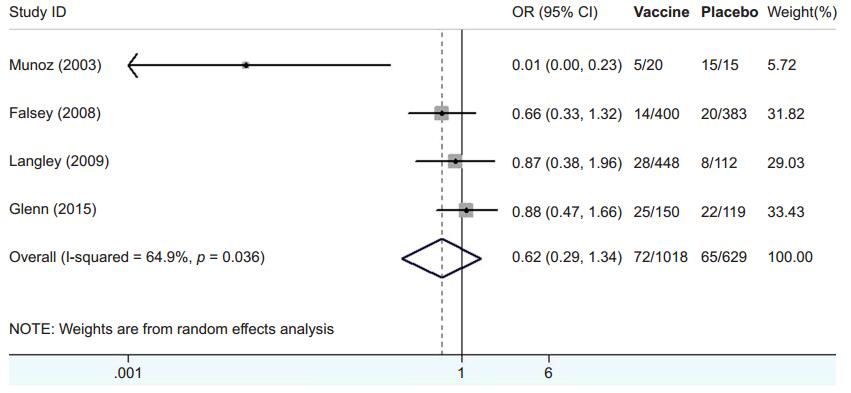
Table 2 shows the characteristics of the 4 articles that examined the effect of an adjuvant. Additionally, Figure 3 summarizes the results for the effect of vaccines with or without the adjuvant. The observed OR was 0.62 (95% CI, 0.29–1.34) and significant effects (p < 0.0001) were observed for both RSV vaccines. The forest plot of the distribution of ORs is shown in Figure 3. Based on these results, vaccines with adjuvant have no significantly different with those without adjuvant. They couldn't accordingly reduce the incidence of RSV infection. The decreased immunogenicity of alum-containing sanofi-pasteur vaccine was observed by Falsey (2008) and Langley (2009) as their OR were 0.66 (95%CI, 0.33–1.32) and 0.87 (95%CI, 0.38–1.96), respectively. The similar result was gained in RSV F nanoparticle vaccine evaluated by Glenn (2015) in which OR was 0.88 (95%CI, 0.47–1.66). Only Munoz's research (2003) showed that PFP-2 with adjuvant had less side effects than that without adjuvant (OR = 0.01, 95%CI: 0.00–0.23). However the reason for the inferior immunogenicity of vaccines adjuvanted with aluminum phosphate is unclear.

Table 2. Studies included in the meta-analysis of vaccines used with/without adjuvant.
-
Egger's test was used to check for publication bias. There was no evidence for publication bias based on funnel plots (p = 0.090). The funnel plot is shown in Figure 4.
-
For patients with bronchiolitis and pneumonia, we found that RSV subunit vaccines could significantly reduce the incidence of RSV infection, but vaccines with adjuvant therapy also had no significantly different with those without adjuvant. However, this meta-analysis was limited owing to the small number of randomized trials. RSV subunit vaccines must be tested in large field trials to minimize issues regarding the appropriateness of pooling and to ensure a more accurate data analysis to prevent RSV infections.
Study selection and characteristics
Publication bias
Significance and limition
-
We screened 1, 792 publications via database searches and identified 472 studies that merited further review. After applying the predetermined exclusion criteria, 13 articles (in which 11 studies included in RSV vaccine's meta-analysis and 4 studies estimated vaccine's meta-analysis with/without adjuvant) met our inclusion criteria for the final meta-analysis (Figure 1). Based on the ORs, there was a significant difference between the experimental and control treatments for all RSV infections (p < 0.00001). The effects of vaccination with the subunit vaccines PFP-1 and PFP-2 on RSV infection had a publication bias. These clinical trials included only ~300 individuals; accordingly, the size of the test samples was insufficient. Additionally, our meta-analysis included studies that used 2 doses, 20 μg and 50 μg, for PFP-1 and PFP-2. However, there is no significant dose-dependence (Langley et al., 2009).
Our meta-analysis highlights the tremendous progress that has been made in the development of RSV vaccines in the past decade. Five candidate vaccine types have been evaluated in clinical trials to date, including subunit vaccines for the immunization of the elderly and pregnant women as well as RSV-seropositive children at high risk of severe RSV disease, and live attenuated vaccines used primarily for the immunization of infants and the elderly (Piedra et al., 1998). Three purified fusion protein (PFP-1, PFP-2, and PFP-3) vaccines have been tested in RSV-seropositive children and have been shown to be safe and reasonably immunogenic (Paradiso et al., 1994; Piedra et al., 1995; Falsey et al., 1996; Tristram et al., 1996; Groothuis et al., 1998; Piedra et al., 1998; Piedra et al., 2003).
We found that PFP-1, PFP-2, and PFP-3 were all able to decrease the incidence of severe RSV infection. Additionally, their effects appeared to be highly similar, probably because they are based on the same F protein subunit and have almost no differences in purity. RSV F nanoparticle vaccines and sanofi-pasteur vaccines were well tolerated in healthy women of childbearing age and elderly population, and induced immune responses that may be consistent with protection against RSV disease (Glenn et al., 2015; Falsey et al., 2008; Langley et al., 2009).
Unfortunately, no licensed vaccine exists for the prevention of RSV infections and diseases at present (Bernstein et al., 2012). None of the current vaccine concepts has entered the advanced stages of clinical development (Kaaijk et al., 2013). One or more of these vaccines may prove clinically effective, provided attention is paid to a number of confounding variables during the design of either pediatric or adult vaccine protocols. For example, individuals with poor diets or a poor metabolism (e.g., a vitamin deficiency) may exhibit general defects in immune responses toward respiratory virus vaccines (Rudraraju et al., 2012; Surman et al., 2012). We suggest recruiting thousands of participants to ensure fair vaccine assessment (Falsey et al., 2012).
The affirmative results of this meta-analysis should not promote the consideration of subunit vaccines in populations who were specifically excluded from the trials because enhanced pathology may be observed owing to an imbalance in the immunological response to the vaccine. In rodent models, subunit F glycoprotein or chimeric FG vaccines shared with FI-RSV the properties of enhancement of pulmonary histopathology during subsequent RSV infection. These observations confirmed the need for caution in studies involving the administration of RSV subunit vaccines to seronegative humans (Connors et al., 1992).
The meta-analysis had various limitations that should be taken into account when interpreting the results. First, only published studies were included, and there may be a publication bias. Second, the retrospective nature of a meta-analysis means that it is subject to the methodological deficiencies of the studies included in the analysis. Third, allocation concealment was poorly reported in most of the studies included in the meta-analysis, and only one was adequate in this regard.
-
This work was supported by grants from Natural Science Foundation of China (Grant Ref. 81371797), the key project of Natural Science Research Found of Education Department of Anhui Province (Grant Ref. KJ2012A152) and Natural Science Foundation of Anhui Province (Grant Ref. 1308085MH129). The authors would like to thank Junwei Yan and Liying Wen, Department of Epidemiology and Statistics, Anhui Medical University for the data processing in this Meta-analysis.
-
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.
-
TZ, CZ and SH designed the experiments. TZ, CZ, LY and JC collected documents, stated and sumed up the data. HQ and WL analyzed the data. TZ, CZ and SH wrote the paper. All authors read and approved the final manuscript.
















 DownLoad:
DownLoad: