-
Dear Editor,
Chikungunya virus(CHIKV), a single-stranded RNA virus that belongs to the genus Alphavirus, family Togaviridae, is transmitted by mosquitoes of the genus Aedes(Diptera: Culicidae), predominantly Aedes aegypti and A. albopictus(Staples et al., 2014). CHIKV infection is most often characterized by fever, headache, fatigue, nausea, vomiting, muscle pain, rash, and joint pain(Burt et al., 2012). Since its discovery in Tanzania, Africa, in 1952 by Robinson et al.(1955), CHIKV outbreaks have occurred occasionally in both Africa and Asia(Burt et al., 2012), but recent outbreaks have spread the disease to other parts of the world(Asia, Europe, and islands in the Indian and Pacific Oceans)(Staples et al., 2014). This dramatic change was due to the occurrence in La Reunion Island in 2005 of a mutation(A226V)in the CHIKV envelope 1(E1)glycoprotein gene. It is hypothesized that this mutation enhanced the infectivity of the virus and its transmission by A. albopictus(Tsetsarkin et al., 2007). Studies have shown that the CHIKV E1 mutation places several countries at risk of epidemic circulation, as evidenced by the spread of the mutated CHIKV in 2005 from the Indian Ocean, following which it led to large epidemics in India in Southeast Asia as well as Italy in Europe(Tsetsarkin et al., 2007; Rezza et al., 2007). In West and Central Africa, the disease is maintained in a sylvatic cycle involving wild non-human primates and forest-dwelling Aedes mosquitoes such as A. furcifer-taylori and A. africanus. In Asia, A. aegypti, an anthropophilic mosquito species, has been the most significant vector(Myers et al., 1965). In contrast, for the mutated CHIKV that caused the 2005-2006 epidemic in La Reunion Isl and in the Indian Ocean region, A. albopictus is the main vector(Tsetsarkin et al., 2007).
Although chikungunya epidemiology is increasingly well characterized in industrialized countries, it remains an imperfect science in developing countries such as Cameroon and is often characterized by epidemic reporting(Peyrefitte et al., 2007; Demanou et al., 2010)rather than being considered a part of ongoing population health surveillance programs. Although Cameroon is considered to be endemic for chikungunya, only one strain of the virus has been isolated from this region and studied so far(Peyrefitte et al., 2007); the other studies carried out in Cameroon to date were serological studies to confirm the circulation of this arbovirus(Ndip et al., 2004; Kuninholm et al., 2006; Demanou et al., 2010).
In this report, we present the results of case-based sentinel surveillance of a dengue-like syndrome from March to September 2013 in Cameroon. In the framework of this surveillance, cases of febrile illness, with symptoms such as fever, asthenia, maculopapular rashes, and arthralgia, were reported from three different regions of Cameroon(Center, South, and Littoral)in May 2013. Blood samples were collected from 12 patients who visited medical centers and tested negative for the hyperendemic Plasmodium falciparum using the malaria rapid diagnostic test. These samples were then sent to Centre Pasteur of Cameroon, the national reference laboratory for chikungunya and dengue. The ages of these patients ranged from 5 to 56 years.
For sample processing, viral RNA was extracted directly from these samples using the commercial QIAamp viral RNA Mini Kit(QIAGEN, Germany)according to the manufacturer's instructions. The presence of CHIKV and dengue virus(DENV)genomes was tested by specific real-time reverse transcription PCR(RT-PCR)using the following and respective primers and probe: forward CHIK-F AAGCTYCGCGTCCTTTACCAAG and reverse CHIK-R CCAAATTGTCCYGGTCTTCCT pri-mers, and probe FAM-CCAATGTCYTCMGCCTGGACACCTTT-TAMRA for CHIKV; forward DENUNI-F AAGGACTAGAGGTTA(G/T)AGGAGACCC and reverse DENUNI-R CG(A/T)TCTGTGCCTGGA(A/T)TGATG, and probe FAM-TCTGGTCTTTCCCAGCGTCAATATGCTGTT-TAMRA. Three of the 12 specimens, that is, 13V-2713 from Yaoundé(Center region), 13V-2798 from Kribi(South region), and 13V-2980 from Douala(Littoral region), were positive for CHIKV and none were positive for DENV. A 1, 000-bp-long partial sequence of the E1 gene of CHIKV from each of the three positive samples was then amplified by endpoint RT-PCR using the following primer pair: forward CHIK10145-F, ACAAAACCGTCATCCCGTCTC; reverse CHIK11158-R, TGACTATGTGGTCCTTCGGAGG. The nucleotide sequences of these PCR products were obtained using BigDye terminator version 2.0 chemistry(Applied Biosystems)according to the manufacturer's protocol—for both sense and antisense strands—on an automated ABI PRISMTM 3100 DNA Sequencer(PerkinElmer, Applied Biosystems). The sequences reported in this article have been deposited in GenBank(accession numbers: KJ508819 for 13V-2798, KJ508820 for 13V-2713, and KJ508821 for 13V-2980). The characteristics(date of sample collection, age, and district and region of origin)of the three patients are presented in Table 1.
Laboratory code Patient age
(years)Date of onset Date of sample collection Patient location (city/region) GenBank accession number 13V-2713 31 20/05/2013 21/05/2013 Yaounde/Centre KJ508820 13V-2798 50 23/05/2013 24/05/2013 Kribi/South KJ508819 13V-2980 56 26/05/2013 26/05/2013 Douala/Littoral KJ508821 Table 1. Characteristics of the three positive samples collected in May 2013 from patients with clinically diagnosed chikungunya in Cameroon.
For sequence analysis, a sequence of 897 nucleotides from the three patients was compared to reference sequences comprising all known genotypes and clades(Figure 1). Consensus sequence editing and multiple sequence alignments were performed with CLC Main Workbench 5.7.2 software(CLC bio, Aarhus, Denmark). Phylograms were inferred both by the distance and maximum likelihood methods implemented in MEGA, version 5.10(Tamura et al., 2011). The evolutionary distances were computed with the Kimura two-parameter algorithm, and the tree was reconstructed by the neighbor-joining method with the Kimura two-parameter method for computing evolutionary distances(Kimura, 1980). Alignment gaps were removed from the analysis for each sequence pair. The reliability of the tree topology was estimated by bootstrap analysis with 1, 000 pseudoreplicate data sets.
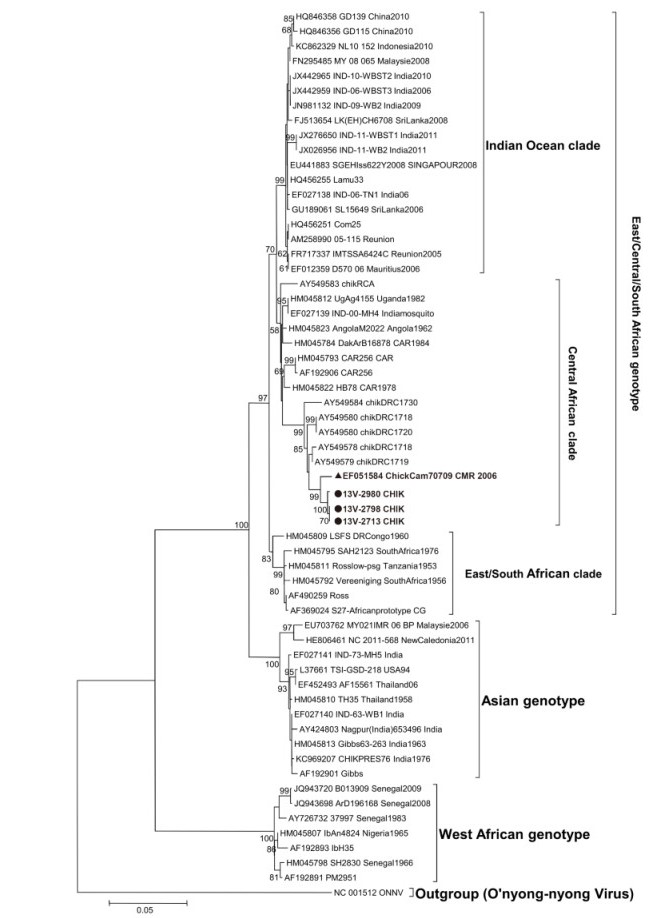
Figure 1. Phylogenetic tree of chikungunya virus (CHIKV) strains, based on the 897 nucleotides corresponding to partial sequences of the E1 gene (nucleotide positions 10222-11119, with respect to the reference strain S27-African prototype). Viruses have been labeled using the GenBank accession number and country and year of isolation where available. Boldface indicates strains from Cameroon, including one from a previous study (specified with a triangle) and those from this study (specified with dots). All newly sequenced strains from Cameroon carried the A226V mutation. Numbers represent the bootstrap support obtained for the corresponding branches. The tree was rooted with the O'nyong-nyong virus (GenBank accession no. NC 001512-ONNV). Scale bar at the bottom indicates nucleotide substitutions per site.
Analysis of CHIKV sequences did not show any codon deletion or insertion on comparison with other African CHIKV sequences available in the GenBank database. A high degree of identity was observed when the sequences were compared with those of the strains isolated in 2000 by Pastorino et al. (2004)in the Democratic Republic of Congo(DRC) and in 2006 by Peyrefitte et al. (2007) in Douala(Littoral region, Cameroon), as well as the strain isolated during the 2006-2007 dengue-like outbreak in Gabon(Peyrefitte et al., 2008). In particular, the newly sequenced CHIKV had the A226V mutation in the E1 envelope protein gene. It has been previously reported that this mutation, reported for the first time in Cameroon in 2006(Peyrefitte et al., 2007), is associated with enhanced replication and fitness of CHIKV in highly anthropophilic and geographically widespread A. albopictus(Tsetsarkin et al., 2007).
The sequence identity among these isolates highlights their common origin and the relative genetic stability of CHIKV in Central Africa despite a gap of several years(more than a decade) and the geographic distance from the DRC outbreaks. As shown in the phylogenetic tree(Figure 1), the CHIKV from Cameroon clustered with CHIKV isolates from DRC with a high bootstrap value of 99. This genotype of CHIKV was found to be closely related to strains from the Central African Republic and the 1982 Uganda isolate. The close genetic relationship suggests continuous circulation of a homologous CHIKV population in Central Africa with a high degree of genetic stability.
Our results indicate that CHIKV strains circulating in these three regions of Cameroon have a common origin and do not differ from strains circulating in Central Africa as they all clustered in the central African lineage. The presence of the E1-A226V mutation indicates the possible virulence of the Cameroonian strains and might explain the emergence or re-emergence of chikungunya in Central Africa during the last decade after the introduction of A. albopictus.
HTML
-
This study was supported by the Department of International Affairs of the Institut Pasteur International Network(ACIP A12-2011). The authors declare that they have no conflict of interest. This study was approved by the National Ethical Committee of Cameroon(permit number, 255/CNE/SE/2011). Written consent was obtained from the patients involved in the study. We wish to thank Magalie Mazelier for her technical assistance.














 DownLoad:
DownLoad: