HTML
-
Innate recognition of viral proteins is an important component of the immune response to viruses. The complement lectins in human serum are important innate immune molecules. Complement contributes to initial defense against viral infections through a sequential protein activation cascade (Sarma et al., 2011). There are three complement activation pathways: the classical, the alternative, and the lectin pathway (Lu et al., 1998; Hoffmann et al., 1999). Mannan-binding lectin (MBL) and ficolins are two kinds of complement lectins. They are able to recognize microbial carbohydrates, and thereby activate the lectin complement pathway by a mechanism similar to the classical pathway, but they use MBL associated serine proteases (MASPs) instead of C1r and C1s.
Three human ficolins have been described: ficolin-2/L-ficolin (Endo et al., 1996), ficolin-1/M-ficolin (Ichijo et al., 1991; Lu et al., 1996), and ficolin-3/Hficolin (Sugimoto et al., 1998), and two types of ficolins have been identified in mice. Each ficolin monomer comprises two functionally important domains, a collagenlike domain (CLD) containing Gly-Xaa-Yaa repeats, and a globular fibrinogen-like domain (Matsushita et al., 1996; Hummelshøj et al., 2007). The fibrinogen-like domain recognizes specific pathogen-associated carbohydrates, and the CLD is responsible for signaling to induce an immune response via MASPs (Matsushita et al., 1996; Hummelshøj et al., 2007). The fibrinogen-like domain of ficolin-2 forms a globular structure like the carbohydrate recognition domain (CRD) of MBL. Ficolin-2 is primarily expressed in the liver and subsequently released into the blood stream. Ficolin-2 is structurally similar to C1q, MBL and lung surfactant proteins A and D (SP-A and SP-D), but the sugar ligand specificities for ficolin-2 and MBL are different (Atkinson et al., 2004).
Human immunodeficiency virus (HIV) is a major global public health issue. Globally, 1.5 million people died because of HIV infections in 2013, and 2.1 million people were newly infected. Worldwide, there were approximately 35 million people living with HIV at the end of 2013. Sub-Saharan Africa was the most affected region in the world, where 24.7 million people were living with HIV in 2013, and accounted for almost 70% of the new HIV infections (WHO, 2014).
HIV belongs to the subgroup of retroviruses. HIV infection causes destruction of immune cells, which results in susceptibility to a wide range of infections. The most advanced stage of HIV infection is acquired immunodeficiency syndrome (AIDS), which takes a long time (2–15 years) to develop (Granich et al., 2009; Williams et al., 2011). Essentially, two types of HIV have been characterized: HIV-1 and HIV-2. HIV-1 is more virulent and infective than HIV-2 (Gilbert et al., 2003). HIV-1 is a member of the Lentivirus genus, classified in the Retro-viridae family. Each virion consists of an inner core surrounded by a lipid bilayer envelope. The genome of HIV-1 is approximately 9.6 kb, and the major genes encoding structural proteins are located at the 5'-end of the genome in the order Gag, Pol and Env. The Env gene encodes a 160-kDa envelope protein, which is cleaved by a cellular protease to generate the external gp120 protein and the transmembrane gp41. Ezekowitz et al. first discovered the interactions between MBL and HIV (Ezekowitz et al., 1989). Further studies by Haurum et al. indicated that the binding of recombinant gp120 to MBL activated the complement lectin pathway (Haurum et al., 1993).
Several reports showed that dysfunction or abnormal expression of ficolins may play crucial roles in the pathogenesis of human diseases including: (1) infectious and inflammatory diseases, e.g., recurrent respiratory infections; (2) autoimmune disease, e.g., systemic lupus erythematosus and IgA nephropathy; and (3) preeclampsia (Kilpatrick et al., 2003; Atkinson et al., 2004; Chapman et al., 2007). However, little is known about the role of ficolin-2 in HIV-1 infection. In this study, we provide evidence of the important protective role of ficolin-2 against HIV-1 infection.
-
This study was approved by the Ethics Committees of Wuhan University School of Medicine. Written informed consent was obtained from all participants. The serum samples of 103 HIV-1-positive patients and 57 healthy donors were collected in the Center for Disease Control and Prevention, Wuhan, China, from 2009 to 2012. All patients were previously identified as HIV-1-seropositive by commercial ELISA tests. Patients receiving immunosuppressive regimens or known to be infected with hepatitis B virus, hepatitis C virus, hepatitis D virus or tuberculosis were excluded.
-
A sandwich enzyme-linked immunosorbent assay (ELISA) was used to measure the concentration of serum ficolin-2 according to methods described in previous studies (Liu et al., 2009; Pan et al., 2012; Zhao et al., 2014). Briefly, 96-well ELISA plates were coated with rabbit antificolin-2 polyclonal antibody. After incubation at room temperature for 1 h, the plates were washed three times with 0.2% Tween-20 in phosphate buffered saline (PBS) solution. Subsequently, 100 μL of each fresh serum sample was added within 6 h of harvesting, and incubated at 37 ℃ for 2 h. The plates were washed three times and blocked with 5% bovine serum albumin overnight. Mouse monoclonal anti-human ficolin-2 (GN5, 1:1000 dilution; Hycult Biotech, Netherlands) was then added to each well and incubated at 37 ℃ for 1 h. The plates were washed three times and incubated with 100 μL horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:1000 dilution). Color development was achieved by adding 100 μL tetramethylbenzidine chromogen-substrate (Sigma). The reaction was stopped by adding 100 μL 0.5 mol/L H2SO4, and the OD450 was measured using an ELISA reader. In addition, a standard ficolin-2 curve was derived by using different concentrations of recombinant ficolin-2 protein. Data were generated from at least three independent experiments. All data shown represent the mean±SEM.
-
HEK293T cells were cultured in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 U of penicillin, and 100 μg of streptomycin per mL. Cells were seeded into 6-well plates at a density of 1 ×106 cells per well in 2 mL DMEM without antibiotics or serum. The cells were transfected with 1 μg of HIV-1 JRFL plasmid (HIV-1 gp120 expressing plasmid) (Hu et al., 2005) or pNL4-3 Luc R-Eplasmid (NIH) (firefly luciferase gene inserted into the pNL4-3 nef gene, in the absence of Env and Vpr genes) (Li et al., 2009) using lipofectamine 2000 (Invitrogen, San Diego, CA). After 48 h, the cells were harvested and used for subsequent experiments.
-
Primers for ficolin-2 were designed using Primer 5 soft-ware: the sense primer was 5'-GTACAGCTCATTCCAGGT-3' and the anti-sense primer was 5'-AGTTGATGCCATCCGCA-3'. Total RNA was purified using the RNeasy kit (Qiagen, Germany) according to the manufacturer's instructions. The quantity and quality of RNA were checked spectrophotometrically. Then, the RNA samples were transcribed into cDNA with a reverse transcription kit (Invitrogen, USA). Real-time PCR was performed on a single-color RT-PCR detection system with SYBR green supermix (ABI, USA). The expression levels of ficolin-2 were determined as follows: the threshold cycle values (Ct) of genes of interest were normalized to the Ct value of the GAPDH reference mRNA (ΔCt); then, the normalized mRNA levels were calculated as 2(-ΔCt).
-
293T cells were transfected by HIV-1 JRFL or HIV-1 NL4-3 R-E- plasmids, and the cell lysates were separated by 12% SDS-PAGE and transferred onto PVDF membranes. Fat-free milk powder solution (10%, w/v) was used for blocking. The membrane was incubated with anti-ficolin-2 antibody (G-12) (1:1000; sc-55242, Santa Cruz, USA) for 1 h at room temperature, washed three times, and then incubated with a 1:5000 dilution of HRPconjugated anti-rabbit IgG (Abbkine, USA) and washed three times. The color was developed with ECL western blotting substrate (Pierce, USA).
-
The ficolin-2 cDNA was amplified and cloned in-frame into the BamH I and EcoR I sites of prokaryotic expression vector pGEX-KG to yield plasmid pGEX-ficolin-2, which was transformed into Escherichia coli BL21 (DE3). The GST-ficolin-2 fusion protein was overexpressed in E. coli after induction with isopropyl β-D-1-thiogalactopyranoside (IPTG, 0.1mmol/L, 23 ℃, 6 h). The expressed GST-ficolin-2 protein was purified using Glutathione Sepharose 4B (Amersham Biosciences). Purified GSTficolin-2 protein was determined by SDS-PAGE and western blotting (data not shown).
-
HEK293T cells were transfected with the HIV-1 JRFL plasmid to generate HEK293T cells transiently expressing gp120 (gp120-293T cells). The gp120-293T cell lysate was incubated with 50 μL of GST or GST-ficolin-2 Sepharose beads for 2 h at 4 ℃ with constant mixing, then centrifuge at 500×g for 3 min. The supernatant was discarded and the beads washed four times with PBS. Glutathione (50 μL of 20 mmol/L) was added in 50 mmol/L Tris-HCl (pH 8.0) to elute the protein from the beads. The mixture was centrifuge, the supernatant collected, then ficolin-2 and gp120 were detected by western blottting.
-
To verify the interaction between HIV-1 gp120 and ficolin-2, we tested the binding of recombinant GSTficolin-2 protein to gp120-293T cells. In brief, different concentrations (20, 40 μg/mL) of recombinant GSTficolin-2 or GST (negative control) were incubated with 293T/gp120-293T cells at 37 ℃ for 30 min. Then the cells were washed with PBS, and incubated with polyclonal GST antibody at 37 ℃ for 2 h. The cells were washed and incubated with Phycoerythrin (PE)-labeled anti-rabbit IgG and Fluorescein Isothiocyanate (FITC)-labeled antigp120 antibody at 37 ℃ for 30 min. The percentage of double positive (GST+ and gp120+) cells was analyzed by flow cytometry using a Beckman Coulter EPICS ALTRA Ⅱ.
-
HEK293T cells were co-transfected with HIV-1 pNL4-3R-E- and JRFL plasmids to produce infectious luciferase-containing viruses. The HIV-1 viruses were harvested 48 h post-transfection. TZM-b1 cells (human cervical carcinoma cells stably expressing CD4, CXCR4 and CCR5) or MT-2 cells (human T lymphoma cells) were grown in 96-well plates at 1×104 cells per well, and following 12 h incubation at 37 ℃, the cells were infected with 200 TCID50 of HIV-1 viruses. GST/GST-ficolin-2 proteins (50 μg) or azidothymidine (AZT; 1 μmol/L) were added at the time of infection, and the cells were lysed to measure the luciferase activities using luciferase reagents (Promega), or HIV-1 P24 protein by ELISA.
TZM-b1 cells or MT-2 cells were grown in 96-well plates as previously described, and the HIV-1 viruses (200 TCID50) were incubated with isovolumetric GST/GST-ficolin-2 beads. The cells were infected with virus, then lysed to measure the luciferase activities or HIV-1 P24 protein.
-
HEK293T cells transiently expressing gp120 (gp120-293T cells) were labeled with 2', 7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethylester (BCECF/AM; 2 μg/mL) at 37 ℃ for 30 min and plated in 96-well plates at 1×104 cells/well. 293T cells were used as control cells. A mix of serum from healthy donors was used as a source of complement. Classic complement pathway activation was blocked by diluting the serum in buffer with hypertonic (1 mol/L) NaCl. The endogenous ficolin-2 and MBL of normal human serum were excluded by affinity chromatography on GlcNAc-agarose (Sigma-Aldrich) and MBL-agarose. The labeled cells were incubated with serum (ficolin-2-, MBL-) and different doses (6, 8, 10, 12, 24 μg/mL) of exogenous recom binant GST-ficolin-2 or GST (negative control) at 37 ℃ for 30min. The supernatants were collected and the fluorescence intensity was measured (FIsup). The remaining cells were lysed with cell lysis buffer and the fluorescence intensity was measured (FIlys). Cell cytotoxicity (%)=FIsup/(FIsup + FIlys).
-
HEK293T cells were plated in 6-well plates at a density of 1×106 cells per well and cotransfected with 1μg HIV-1 gp120 expressing plasmid and 0.4 μg pNL4-3-luc-R-E-using lipofectamine 2000. After 72 h, the medium was centrifuged and filtered. The aliquoted virus containing medium was stored at –80 ℃.
-
Peripheral blood mononuclear cells (PBMCs) were separated from the plasma of healthy donors using the Ficoll-Hypaque method (Zhang et al., 2004). Then, CD4+ T cells were isolated via a negative selection principle with a MagCellect Magnet (R & D Systems). Briefly, the PBMC suspension was first incubated with the MagCellect Antibody Cocktail at room temperature for 10 min. After washing with PBS + 1% BSA, the cells were incubated with the MagCellect Streptavidin Ferrofluid. The cell suspension was then placed within a magnetic field. Magnetically tagged cells migrated toward the magnet (this is the unwanted cell fraction), leaving the CD4+ T cells in suspension. The enriched CD4+ T cells were resuspended in RPMI 1640 medium pulsed with 1% FCS and seeded in 24-well plates at a density of 2 ×105 cells per well. The isolated CD4+ T cells were inoculated with HIV-1 pp medium (100 μL/well) and incubated with different concentrations of recombinant GSTficolin-2 or GST (negative control). Twenty-four hours later, cells were lysed to measure the luciferase activities using luciferase reagent.
-
Data were analyzed using SPSS 17.0 software, oneway ANOVA and Student's t-test. Differences were considered statistically significant when P < 0.05; **** denotes P < 0.0001.
Clinical specimens
Measurement of serum ficolin-2 concentration
Cell culture and transfection
Ficolin-2 expression determined by real-time RT-PCR
Ficolin-2 expression determined by Western Blotting
Purification of recombinant ficolin-2 protein
GST pull-down assay
Binding assay of ficolin-2 to HIV-1 gp120
HIV-1 infection protocol
Complement dependent cytotoxicity assay (CDC assay)
Package of HIV-1 pseudovirus (HIV-1 pp)
Isolation of CD4+T cells and HIV-1 pp infection
Statistical data analysis
-
The serum ficolin-2 concentrations of HIV-1 patients were significantly higher than those of healthy donors (****P < 0.0001) (Figure 1A). The mean concentration of ficolin-2 was 9.98 μg/mL in HIV-1 patients, and 4.31 μg/mL in healthy donors. There were no statistically significant differences in age and gender between healthy donors group and the HIV-1 patients group.

Figure 1. HIV-1 infection stimulated the expression of ficolin-2. (A) Increased initial ficolin-2 protein concentrations in HIV-1 patients compared with healthy donors. (B) 293T cells were transfected with HIV-1 JRFL or HIV-1NL4-3R-E-plasmids and the ficolin-2 mRNA expression level was detected by real-time RT-PCR. * indicates P < 0.05* vs. control. (C) 293T cells were transfected with HIV-1 JRFL or HIV-1pNL4-3R-E-plasmids and the ficolin-2 protein expression was detected by western blotting.
-
HEK293T cells were transfected with 1 μg of HIV-1 JRFL plasmid or HIV-1 pNL4-3 Luc R-E- plasmid, and the cells were harvested after 48 h. Ficolin-2 expression was then detected by real-time RT-PCR and western blotting. We detected that HIV-1 JRFL and HIV-1 pNL4-3 Luc R-Ecould significantly induce fiolin-2 expression (Figure 1B, 1C).
-
The ability of ficolin-2 to bind to HIV-1 gp120 was tested in cells transiently expressing HIV-1 gp120. We incubated recombinant ficolin-2 with 293 cells or 293T cells transiently expressing HIV-1 gp120 (gp120-293T cells) and examined the proteins that associated with the cells (Figure 2A). We verified the binding of ficolin-2 and gp120 by GST pull down assay, and found that gp120 could bind with GST-ficolin-2 beads but not with GST beads (Figure 2B). The binding of ficolin-2 to the cells was also detected by flow cytometry. Ficolin-2 could bind with gp120-293T cells but not with 293T cells, and this binding corresponded to ficolin-2 concentrations (Figure 2C). We also found that the binding between ficolin-2 and gp120-293T cells was inhibited by deoxynojirimycin (DNM) (Zhang et al., 2004), a wellknown inhibitor of cellular α-glucosidase Ⅰ-Ⅱ enzymes (Figure 2D). These results suggested that ficolin-2 could bind to HIV-1 gp120.
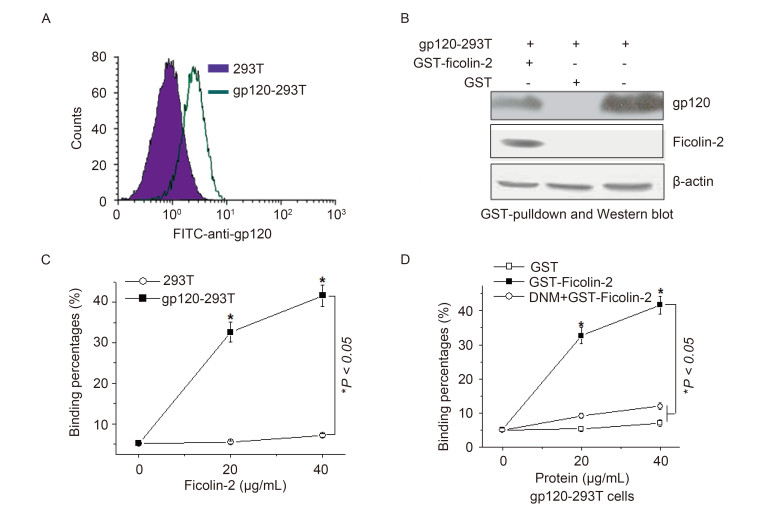
Figure 2. Ficolin-2 recognized and bound to HIV-1 gp120. (A) Analysis of the expression of gp120 on the surface of gp120-expressing cells. (B) The binding between GST-ficolin-2/GST and gp120-expressing cells analyzed by GST-pull down assay. (C) Flow cytometry analysis of the binding between GST-ficolin-2 and 293T cells or gp120-293T cells (gated GST and gp120 double positive cells). (D) Flow cytometry analysis of the binding between GST-ficolin-2/GST and gp120-293T cells (gated GST and gp120 double positive cells) in the presence of DNM. * indicates P < 0.05. All experiments were repeated at least three times.
-
To test whether the binding of ficolin-2 and HIV-1 gp120 could affect HIV-1 infection, we used HIV-1 NL4-3 to infect TZM-b1 cells or MT-2 cells, and detected the luciferase activities or HIV-1 P24 protein. Compared with GST, GST-ficolin-2 significantly inhibited HIV-1 infection. The positive control AZT also significantly inhibited HIV-1 infection (Figure 3A, 3C).
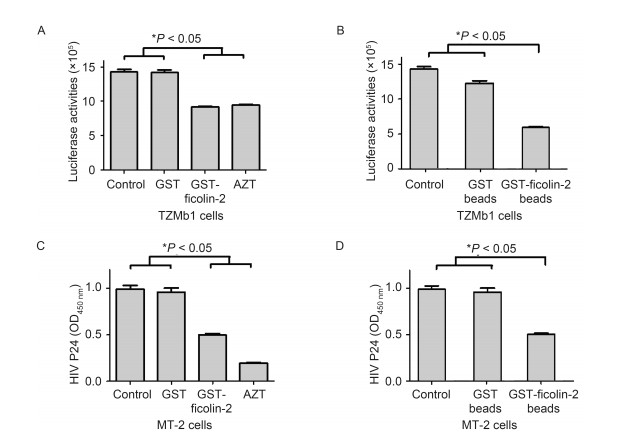
Figure 3. Ficolin-2 inhibited HIV-1 infection. (A) TZM-b1 cells were infected by HIV-1 NL4-3 and the luciferase activities were detected. Compared with GST, GST-ficolin-2 could significantly inhibit HIV-1 infection. AZT was used as a positive control. (B) HIV-1 NL4-3 was first incubated with GST or GST-ficolin-2 beads, then TZM-b1 cells were infected by the virus. Compared with GST beads, GST-ficolin-2 beads could reduce the HIV-1 NL4-3 infectivity. (C) MT-2 cells were infected by HIV-1 NL4-3 and HIV-1 P24 levels were detected. Compared with GST, GST-ficolin-2 could significantly inhibit HIV-1 infection. AZT was used as a positive control. (D) HIV-1 NL4-3 was first incubated with GST or GST-ficolin-2 beads, then MT-2 cells were infected by the virus. Compared with GST beads, GST-ficolin-2 beads reduced the HIV-1 NL4-3 infectivity. * indicates P < 0.05. All experiments were repeated at least three times.
Subsequently, HIV-1 NL4-3 was incubated with GSTficolin-2/GST-Sepharose beads; the supernatants were collected after GST-ficolin-2 Sepharose beads adsorbed the virus, and used to infect TZM-b1 or MT-2 cells. Then luciferase activities or HIV-1 P24 proteins were detected. We found that HIV-1 NL4-3 infection significantly decreased after GST-ficolin-2-sepharose beads adsorbed the virus compared to GST beads and controls (Figure 3B, 3D).
-
To examine the ability of ficolin-2 to activate the complement lectin pathway through binding to HIV-1 gp120, we detected whether recombinant ficolin-2 possessed the ability to induce CDC using gp120-293T cells as the target. In the presence of exogenous recombinant GST-ficolin-2, MBL and ficolin-2 deficient serum significantly induced CDC toward gp120-293T cells. GST-ficolin-2 induced CDC in a dose-dependent fashion compared with mock cells, however GST could not induce CDC toward gp120-293T cells, which suggested the effect was induced by ficolin-2 only (Figure 4A).
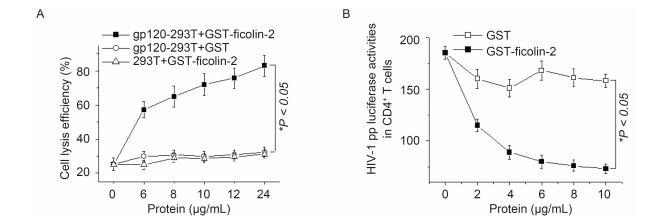
Figure 4. Ficolin-2 inhibited HIV-1 pp infection and induced CDC activities. (A) Compared with GST, GST-ficolin-2 could significantly induce complement dependent cytotoxicity (CDC) toward gp120-293T cells, but not 293T cells. All the experiments were repeated at least three times. (B) CD4+ T cells were infected by HIV-1 pp and the luciferase activities were detected. Compared with GST, GST-ficolin-2 could significantly inhibit HIV-1 infection in a dose dependent manner. * indicates P < 0.05. All experiments were repeated at least three times.
-
Next, we used HIV-1 pp carrying luciferase to infect CD4+ T cells in the presence of GST or GST-ficolin-2 proteins. Compared with GST, the luciferase activities declined on GST-ficolin-2 treatment (Figure 4B). This result suggested ficolin-2 could inhibit HIV-1 entry into CD4+ T cells, and the effect was ficolin-2 dose-dependent.
Increased serum ficolin-2 protein concentrations in HIV-1 patients
HIV-1 stimulated the expression of ficolin-2
Binding of recombinant ficolin-2 protein to HIV-1 gp120
Recombinant ficolin-2 inhibits HIV-1 infection
Recombinant ficolin-2 binds to gp120 and induces CDC activities
Recombinant ficolin-2 inhibits HIV-1 pp infection
-
Mannan-binding lectin and ficolins represent components of the lectin complement pathway (Endo et al., 1996; Sugimoto et al., 1998; Liu et al., 2005). They are soluble oligomeric proteins with lectin-like activity, and they can recognize pathogen-associated molecular pattern (PAMP) carbohydrate molecules on the surfaces of pathogens, and apoptotic, necrotic and malignant cells (Holmskov et al., 2003; Honore et al., 2010; Ren et al., 2014). MBL levels and MBL-mediated complement activation increase in HIV infected patients, which suggested the lectin pathway is involved in HIV infection (Lars et al., 2003). MBL gene polymorphisms determine serum MBL levels among advanced HIV-infected patients (Garred et al., 2006). MBL can bind to HIV-1 envelope proteins gp120 and gp41 and activate complement. This complement activation initiated by the lectin pathway affects the clearance of HIV-1 from the blood and enhances antibody-mediated neutralization (Ying et al., 2004).
The main objective of this study was to explore the role and immunological mechanism of ficolin-2 during HIV-1 infection. We discovered the serum ficolin-2 level in HIV-1 patients was significantly higher than that in healthy controls (Figure 1). We also found HIV-1 infection could stimulate the expression of ficolin-2 (Figure 1). HIV gp120 is highly glycosylated with half of the 120 kDa molecular mass consisting of N-linked glycans (Li et al., 2008). Mounting evidence has revealed that the N-linked glycans of HIV gp120 can inhibit effective adaptive immune responses to HIV (Wyatt et al., 1998). We hypothesized that the increase of serum ficolin-2 on infection was associated with binding to HIV-1 gp120. To determine the interaction of HIV-1 gp120 with ficolin-2, HIV-1 gp120 expressing cells were incubated with recombinant ficolin-2. We found that ficolin-2 could bind to HIV-1 gp120, and the glycosylation inhibitor DNM could inhibit the binding (Figure 2).
Saarloos et al. (1995) reported that HIV infection of a T cell line increased the spontaneous complement activation when exposed to normal human serum, but the activation did not correlate with levels of MBL in serum from a panel of normal donors. Based on our discoveries, we speculated that the increase in ficolin-2 may be involved in the activation of the complement pathway. We found that recombinant ficolin-2 could promote complement dependent cytotoxicity of cells expressing gp120 in a ficolin-2 dose-dependent manner (Figure 4A). This result suggested that HIV-1 gp120 expressed on CD4+ T cells may bind to ficolin-2 and activate the lectin pathway.
HIV delivers genetic material into target cells by the fusion of the viral membrane and the plasma membrane of the host cell. This fusion is mediated by the glycoproteins gp120 and gp41 present on the viral envelope (Dimitrov et al., 1995). The envelope glycoproteins also mediate the binding of HIV to antigen presenting cells through mannose receptors (Trujillo et al., 2007). We speculated that agents interacting with viral envelope glycans might disturb the efficient interaction between HIV-1 gp120 (or gp41) and its (co)receptors through steric hindrance. We tested the interaction of ficolin-2 and HIV-1 gp120. Then we infected target cells (TZM-b1 and MT-2 cells) with HIV-1, and found that ficolin-2 could inhibit HIV-1 infection (Figure 3). We also inoculated CD4+ T cells with HIV-1 pp-LUC; the luciferase activities of the cell lysate show the level of infection of target cells by HIV-1 pp. With increasing amounts of recombinant ficolin-2, infection by HIV-1 pp decreased gradually (Figure 4B). These findings indicate that ficolin-2 blocked the entry of HIV-1 into cells and induced lysis of HIV-1 infected cells.
More and more, lectins are emerging as promising anti-viral agents as an alternative approach to neutralizing antibodies, which suffer from the tedious and time consuming nature of the selection of efficient monoclonal antibodies and the weak immunogenicity of viral envelope glycoproteins. Lectins can target several glycan moieties at once, making it difficult for the virus to adapt rapidly. For example, the virucidal protein cyanovirin-N, as an oligomeric lectin, is highly effective in neutralization of particular envelope viruses such as HIV, influenza and vaccinia (Keeffe et al., 2011).
In conclusion, this study suggests that ficolin-2 plays an important role in blocking HIV-1 entry into cells through interaction with envelope glycoprotein gp120. The results may have particular significance in the light of the application of lectin affinity plasmapheresis in dialysis patients, and as an alternative antiviral agent to ficolin-2, a promising agent for the treatment of HIV and HCV (Zhao et al., 2014; Tullis et al., 2009). This report also provides a new therapeutic strategy for the treatment of AIDS through the lectin complement pathway.
-
The authors declare that they have no conflict of interests. This article does not contain any studies with human or animal subjects performed by any of the authors.
-
This work was supported by grants from the National Natural Science Foundation of China (31221061, 31270176, 31370197 and 21572173), National Outstanding Youth Foundation of China (81025008), the Hubei Province's Outstanding Medical Academic Leader Program and Innovation Team Program (523-276003 and 2015CFA009) and the Wuhan Applied Basic Research Project (2015060101010030).
-
XLZ designed the experiment. FLL, JL, XHS, YNZ, TLC and RGY did the experiments. TLC provided samples. XLZ and FLL wrote the manuscript.














 DownLoad:
DownLoad: