HTML
-
Mammalian cells are encased by a selectively permeable membrane, which is composed of lipids and proteins and separates the cellular cytoplasm from its surroundings (Goldberg and Riordan, 1986). The cell membrane proteins usually contain carbohydrate residues directed towards the exterior and are important for the interaction of cells with each other and with external proteins (Goldberg and Riordan, 1986). To maintain proper homeostasis, the protein and lipid components of the cell membrane are finely controlled in a dynamic and responsive state. It has been widely demonstrated that the external (plasma) membrane is often damaged in a wide range of human diseases, which compromises the integrity of the cells (Goldberg and Riordan, 1986). Due to the host cell membrane plays a crucial role in defense, particularly serving as an initial barrier for pathogen infection, it has been a target frequently hijacked by pathogens to invade host cells (Chakraborty et al., 2012). In virus-associated diseases, it is also very common for changes in the composition and function of proteins within the host cell membrane to occur during viral pathogenesis (Chakraborty et al., 2012).
Epstein-Barr virus (EBV or HHV-4) and Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) are members of the human γ-herpesvirus subfamily and are lymphotropic viruses in natural or experimental settings (Epstein et al., 1964; Chang et al., 1994). Like other herpesviruses, they are capable of establishing latent infection in host cells and reactivating for lytic replication under certain conditions. EBV has been linked with many diseases in humans, including infectious mononucleosis (IM), Burkitt's lymphoma (BL), nasopharyngeal carcinoma (NPC), Hodgkin's disease (HD) and T-cell lymphoma; while KSHV has been associated with Kaposi's sarcoma (KS), primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD) (Cesarman et al., 1995; Mesri et al., 2010). It has been documented that EBV can infect B cells, epithelial cells and some T cells (Spear and Longnecker, 2003; Calderwood et al., 2007). In contrast, it seems that KSHV has a broader range of cell tropism and is able to infect B cells, endothelial cells, epithelial cells and cells of monocyte or macrophage lineage in vitro (Veettil et al., 2014). Although the cell tropism and pathogenesis of these two oncogenic viruses differ, increasing evidence has shown that they exploit similar strategies to invade host cells during their latent and lytic life cycles, particularly in induction of host cell proliferation, transformation and tumorigenesis, and escape from host antiviral responses (Riethmüller et al., 2006).
Given the important roles of the host cell membrane in cellular metabolism, homing, communication and especially immune surveillance, it is not surprising that both EBV and KSHV have evolved specialized proteins that manipulate the composition and function of host cell membrane proteins to avoid host antiviral immune responses and establish persistent infection (Chazal and Gerlier, 2003; Heaton and Randall, 2011; Mazzon and Mercer, 2014). Deciphering how EBV and KSHV hijack the host cell membrane will help to provide new insights into understanding the mechanisms of cell transformation and oncogenesis induced by other tumor-inducing viruses. Indeed, open reading frame (ORF) analysis from viral genome sequences has revealed that both EBV and KSHV encode several important membrane-associated proteins, which include regulators of cell transformation and signaling, modulators or mimics of cellular receptors, and viral cytokines and chemokines, to directly or indirectly take over host cell membrane signaling (Figure 1). In this review, we summarize the functional similarities of the viral proteins encoded by EBV or KSHV for manipulating the composition of host cell membranes and signaling for viral pathogenesis, and highlight the recent evidence for how EBV and KSHV similarly or differently utilize these membrane-associated proteins to facilitate their latent and lytic infection.
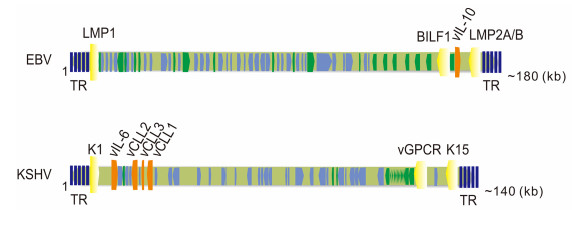
Figure 1. Genomic localization of the host cell membrane proteins, cytokines and chemokines en-coded by EBV and KSHV. Host cell membrane proteins are shown in yellow; cytokines or chemokines are indicated in orange. Latency-asso-ciated genes are in green; lytic genes are in light blue. TR, terminal repeats.
-
Increasing evidence indicates that the herpesviruses, including EBV and KSHV, usually encode several proteins for cell transformation and signaling to protect and preserve the virus-infected cells. Among these proteins are unique viral membrane proteins that have no amino acid sequence similarity to cellular proteins. For instance, Latent membrane protein 1 (LMP1) and K1 are viral oncoproteins encoded by EBV and KSHV, respectively. Although these two genes have highly divergent sequences, the function of the gene products seems to be conserved.
Extensive studies have revealed that the LMP1 protein contains six transmembrane-spanning domains and a 200 amino acid cytoplasmic domain at the carboxyl terminus (Wang et al., 1985; Wang et al., 1988). The carboxyl terminus of LMP1 can be divided into two functional motif regions–CTAR1 and CTAR2. LMP1 is expressed in latently infected B cells and can be upregulated during the lytic replication cycle in epithelial and B cells. It has been shown that LMP1 can transform fibroblasts in vitro and mimics the B-cell activation antigen CD40. Similar to CD40 and other receptors, CTAR1 and CTAR2 in the cytoplasmic domain of LMP1 are associated with several essential proteins including the tumornecrosis factor (TNF) receptor-associated molecules TRAF and TRADD, for activation of the nuclear factor (NF)-κB pathway and for EBV-induced immortalization of B lymphocytes (Izumi et al., 1997). However, distinct from CD40, the transduction of LMP1 signals is not due to extracellular ligands or cross-linking but is caused by multimerization of LMP1 through its transmembrane domains, which mimics ligand-induced CD40 receptor aggregation (Figure 2A). Moreover, this multimerization leads to constitutive activation of NF-κB, cJun N-terminal kinase (JNK) and phosphoinositide 3-kinase (PI3K) activity, as well as induction of expression of many downstream genes including Bcl2 for cell survival (Hatzivassiliou et al., 1998). Hence, LMP1 mainly functions as a B-lymphocyte CD40 receptor to contribute to EBV-infected cell growth, survival and transformation. Interestingly, recent studies in animal models have shown that EBV with LMP1 deficiency can still establish long-term viral latency in vivo, but is unable to induce lymphomas (Gujer et al., 2015; Ma et al., 2015), and LMP1 likely supports the survival of EBV-infected cells in a competitive environment that favors differentiation into memory B cells rather than plasma cells (Thorley-Lawson et al., 2013). Importantly, LMP1 is also able to promote cell migration and globally regulate chromatin and expression of genes including tumor suppressor DOK1 (Siouda et al., 2014), pro-inflammatory cytokines (Xiao et al., 2014), and small non-coding RNA (Motsch et al., 2007; Amort et al., 2015). The fact that LMP1 is present in exosomes from EBV-infected B cells and NPC cells indicates that LMP1 not only plays a role in immune modulation but also in cell communication and tumor microenvironment homeostasis (Meckes et al., 2010).
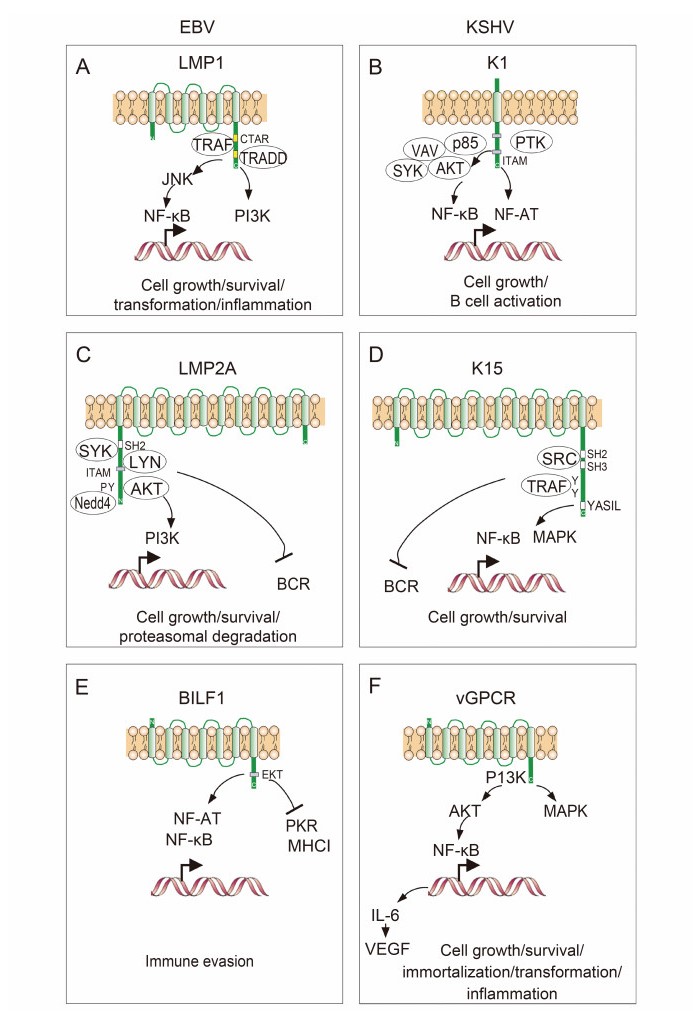
Figure 2. Structure of the host cell membrane proteins encoded by EBV and KSHV. (A–F) LMP1 and K1, LMP2A and K15, and BILF1 and vGPCR are encoded individually by EBV and KSHV, respectively. Interactions with cellular partners and activation of cellular signaling pathways are indicated.
Similar to the LMP1 of EBV, KSHV encodes an ORF called K1 (Lagunoff and Ganem, 1997; Lee et al., 1998b). In contrast to LMP1, however, K1 is a single transmembrane glycoprotein (Figure 2B) (Zong et al., 1999). Although the amino-terminal domain of K1 is highly variable, the carboxyl-terminal cytoplasmic domain is relatively well conserved. Moreover, this carboxyl terminus contains a functional immunoreceptor tyrosinebased activation motif (ITAM), which is important for cellular activation (Lee et al., 1998a; Lagunoff et al., 1999). Interestingly, similar to LMP1, K1 appears to be constitutively activated independently of the ITAMbased signal transduction of ligand-receptor interaction and is also able to transform fibroblasts in vitro (Lagunoff et al., 1999). In addition, it has also been shown that K1 interacts with several cellular proteins including p85, Vav, Syk and Akt kinases to activate NF-κB and NF-AT pathways for cell growth and B-cell activation (Bowser et al., 2002; Tomlinson and Damania, 2004; Tomlinson and Damania, 2008). In contrast with LMP1, K1 has been shown to induce angiogenesis and cell invasion through promoting the secretion of vascular endothelial growth factor (VEGF) and MMP-9 (Wang et al., 2004) and to transform endothelial cells via activation of the PI3K pathway (Wang et al., 2006), as well as functioning in anti-apoptosis and endocytosis (Tomlinson and Damania, 2008; Wen and Damania, 2010). Interestingly, Damania's group showed that K1 was also highly upregulated by the master regulator of lytic replication, RTA, and in turn augmented viral lytic replication (Bowser et al., 2006; Zhang et al., 2016).
-
The activation of immune cells through a receptor at the membrane surface is important for antiviral immune responses, and is often targeted by viral infection. During EBV latent infection, LMP2A is highly expressed in B cells for impairing B-cell activation (Fruehling and Longnecker, 1997; Caldwell et al., 1998). It has been demonstrated that LMP2A has 12 transmembrane domains and short amino-terminal and carboxyl-terminal domains (Figure 2C). There are three tyrosine-based SH2 domain binding sites located in the amino-terminal cytoplasmic region, two of which form a functional ITAM (Fruehling and Longnecker, 1997), which is required for LMP2A to associate with Lyn, Syk and Csk kinases (Burkhardt et al., 1992). In contrast to LMP1, although LMP2A is dispensable for EBV-induced immortalization of B cells, it can block normal B-cell receptor (BCR) signaling in EBV-negative B cells (Miller et al., 1993). This indicates that LMP2A may play a significant role in the establishment and maintenance of EBV latency (Miller et al., 1994; Miller et al., 1995). Many regulatory functions of LMP2A on host cell proliferation, activation, migration, transformation and survival have been reported and were recently summarized by Cen and Longnecker (2015). For instance, the recruitment of Syk and Akt kinases is required for LMP2A to transform and increase migration of epithelial cells, which relies on its ITAM motif (Lu et al., 2006; Fotheringham et al., 2012; Fukuda and Kawaguchi, 2014). In LMP2A transgenic mice, LMP2A could also activate constitutive signals for B cell survival through ITAM/Syk, Ras/PI3K/Akt and mitogen-activated protein kinase (ERK/MAPK) pathways (Merchant et al., 2000; Portis and Longnecker, 2004; Fukuda and Longnecker, 2005; Anderson and Longnecker, 2008). In addition, LMP2A can block the expression of LMP1 to indirectly inhibit interleukin (IL)-6 expression through the Janus kinase/signal transducers and activators of transcription (JAK-STAT) pathway (Stewart et al., 2004), and can drive cell progression by reducing the expression level of p27 (Fish et al., 2014). Furthermore, it has been shown that LMP2A is highly expressed in the EBV latency types Ⅱ and Ⅲ, including EBV-immortalized B-lymphoblastoid cell lines (LCLs), Hodgkin's and nonHodgkin's lymphoma, other lymphoproliferative malignancies, as well as NPC and gastric carcinoma (Qu et al., 2000; Fox et al., 2010; Han et al., 2012). These discoveries indicate that LMP2A may provide a potential therapeutic target for the treatment of EBV-associated cancers.
In the equivalent position to the EBV LMP2A, KSHV encodes a distinct ORF named K15 (Glenn et al., 1999). It has been demonstrated that K15 contains 4–12 transmembrane domains and a short amino-terminal cytoplasmic domain (Figure 2D). In contrast to LMP2A, K15 is weakly expressed in KSHV-latently infected primary effusion lymphoma (PEL) cells, while levels are significantly increased upon stimulation with chemicals such as 12-O-tetradecanoyl-phorbol-13-acetate (TPA). Similar to LMP2A, different variants of K15 exist; however, the signaling motifs (including SH2 and SH3 binding motifs and a YASIL sequence) located in the cytoplasmic domain of K15 are highly conserved, and the tyrosine residue within the putative SH2 binding motif is constitutively phosphorylated. Like LMP2A, K15 is capable of blocking BCR signal transduction, although it is unable to elicit cellular signal transduction upon antibody stimulation. This could explain to a certain extent why LMP2A but not K15 is highly expressed during viral latency. Interestingly, K15 was exclusively found to induce angiogenesis and invasiveness of endothelial cells (Bala et al., 2012; Gramolelli et al., 2015). In addition, it was demonstrated that several intracellular signaling pathways including MAPK and NF-κB were activated by K15 to induce inflammation and angiogenesis (Brinkmann et al., 2003; Brinkmann et al., 2007; Wang et al., 2007; Pietrek et al., 2010).
-
In addition to encoding a viral modulator of host cell membrane signaling, EBV and KSHV also express proteins that mimic host cell membrane receptors. G-protein-coupled receptors (GPCRs) are a diverse family of membrane receptors that can be activated by a variety of ligands (Rosenbaum et al., 2009). All GPCRs contains seven transmembrane domains and couple to hetero-Gproteins in response to receptor activation (Rosenbaum et al., 2009). EBV and KSHV, as oncogenic members of the herpesvirus family, are particularly successful at evading or subverting the host immune response and have been shown to hijack GPCRs to favor their latent and lytic infection. In KSHV, the viral mimicker of cellular GPCR (vGPCR) is encoded by ORF74 (Figure 2F) and displays constitutive activity correlated with oncogenesis in vitro and in vivo (Arvanitakis et al., 1997; Bais et al., 1998; Jham and Montaner, 2010). In transgenic mice, vGPCR was shown to activate MAPKs, phospholipase C (PLC), PI3K and Akt (Smit et al., 2002), as well as NF-κB via the PI3K and Akt pathway (Pati et al., 2001), and subsequently release IL-6 and stimulate VEGF production in both paracrine and autocrine manners for cell growth and survival (Pati et al., 2001).
EBV also encodes a single viral GPCR, called BILF1, in the lytic replication phase (Figure 2E). Similar to vGPCR from KSHV, BILF1 is also able to transform cells in vitro and leads to tumor formation in vivo (Lyngaa et al., 2010), which may be dependent on a pattern of constitutive signaling through the GαI-activated pathway that in turn contributes to viral lytic replication (Paulsen et al., 2005). In the context of immune evasion, it has been shown that BILF1 can constitutively inhibit the phosphorylation of protein kinase R (PKR) (Beisser et al., 2005), and can activate transcriptional factors of NF-κB and NF-AT to prevent the host cell antiviral response (Spiess et al., 2015). In addition, BILF1 reduces the levels of major histocompatibility complex (MHC) class Ⅰ at the cell surface through the lysosomal degradation pathway (Zuo et al., 2009). Further studies have revealed that this function of BILF1 is dependent on the EKT signal motif located in its cytoplasmic tail (Zuo et al., 2011; Griffin et al., 2013). Similar to other viral GPCRs, BILF1 is able to hetero-oligomerize with other chemokine receptors like CXCR4, although BILF1 was previously demonstrated to be an orphan GPCR (Nijmeijer et al., 2010). These findings indicate that both GPCR homologs, encoded by EBV and KSHV, have highly similar functions in redirecting cellular GPCR signaling to favor for viral lytic replication.
-
In addition to encoding their own viral membrane-associated proteins, it has been revealed that the genomes of EBV and KSHV also encode one or more viral cytokines or chemokines (Figure 1), allowing them to fully take over host membrane protein signaling. These viral genes have distinct homology to their cellular counterparts and could modulate cell signaling pathways to promote survival of the infected cell and escape from host antiviral immune surveillance. For example, EBV encodes a viral IL-10 (vIL-10) cytokine that is homologous to human IL-10 (Figure 3, left panel) and is expressed during primary or reactivated EBV infection phases for induction of B-cell transformation and growth (Miyazaki et al., 1993; Stuart et al., 1995; Bejarano and Masucci, 1998). To further prevent activation and recognition of T cells, vIL-10 has been found to downregulate the expression of the transporter protein TAP1, which associates with MHC class Ⅱ antigens presented on monocytes, macrophages and B cells with EBV infection (de Waal Malefyt et al., 1991; Zeidler et al., 1997; Jochum et al., 2012), and also block interferon (IFN)-γ synthesis for enhancing EBV-infected B cell survival (Swaminathan et al., 1993; Suzuki et al., 1995). Interestingly, it has been shown that vIL-10 is highly conserved in various EBV isolates from gastric carcinomas and NPCs (Kanai et al., 2007; Chao et al., 2011) and may contribute to acute infection of permissive host cells by facilitating survival and dissemination of EBV-infected cells as seen in a murine herpesvirus model (Lindquester et al., 2014).
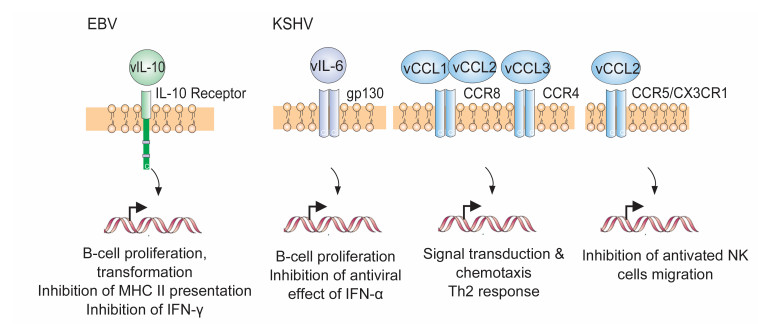
Figure 3. Schematic representation of the cytokines/chemokines encoded by EBV and KSHV and their related receptors and downstream signaling events.
Although KSHV does not encode an ORF similar to the EBV vIL-10, a viral cytokine named vIL-6 is encoded. It has been demonstrated that vIL-6 is secreted from B cells after KSHV infection (Moore et al., 1996; Neipel et al., 1997; Nicholas et al., 1997) and can induce cellular IL-6 expression to promote cell proliferation of PEL cells (Mori et al., 2000) and block the antiviral effects of IFN-α (Chatterjee et al., 2002). However, distinct from cellular IL-6, vIL-6 only requires the gp130 subunit instead of both gp130 and IL-6α receptors for signal transduction (Figure 3, middle panel) (Molden et al., 1997). Unexpectedly, in the context of KSHV-associated diseases, vIL-6 is highly expressed in PEL and MCD cells (Parravicini et al., 1997; Jones et al., 1999), but rarely in KS cells (Staskus et al., 1999; Parravicini et al., 2000).
Since chemokines play a profound role in leukocyte trafficking and development of adaptive immune responses, in addition to cytokines, KSHV also encodes several viral gene products with homology to cellular chemokines, known as vCCL1, vCCL2 and vCCL3 (Figure 3, right panels) (Boshoff et al., 1997). Unlike their cellular counterparts, vCCL1 and vCCL2 bind efficiently to the CCR8 receptor (Sozzani et al., 1998), while vCCL3 activates the CCR4 receptor for signal transduction and chemotaxis in monocytes (Stine et al., 2000). It has been also proposed that vCCL2 and vCCL3 may play an immunomodulatory role in directing inflammation from the Th1 to Th2 type response for KSHV immune evasion (Sozzani et al., 1998; Singh et al., 2004). Recent studies further showed that vCCL2 is the only one of the four cytokine/chemokines encoded by KSHV that binds to natural killer (NK) cells through two different receptors, CX3CR1 and CCR5, which inhibits the migration of activated NK cells (Yamin et al., 2013). In contrast to KSHV, no viral counterpart of cellular chemokines in the EBV genome has been revealed so far. This indicates that there is a significant difference between EBV and KSHV in manipulating host cytokine and chemokine signals, and could explain why EBV and KSHV have different cell tropisms for infection, albeit both of them infect B cells.
-
Recent progress in the tumor virology of both EBV and KSHV has given rise to important concepts relating to changes in the composition and function of host cell membrane proteins. It has been demonstrated that both EBV and KSHV have evolved a diverse array of viral genes to manipulate the host cell membrane, which encode unique viral proteins, viral homologues of cellular proteins and proteins involved in cell membrane signaling. The strategies utilized by these two viruses lead to deregulation of normal cellular pathways including apoptosis, antiviral immune surveillance and arrest in cell growth. As both EBV and KSHV have tropisms for different cell types, a certain subset of viral proteins located in the cell membrane might function in a cooperative manner to aid virus survival in different cellular en vironments, to ensure a life-long persistent infection within the host cells. In view of the fact that individual proteins of EBV or KSHV may or may not appear concurrently, the strategies that are used to subvert the host cell antiviral immune response and override cell-cycle checkpoints for cell growth or survival are often concordant. For instance, LMP1, encoded by EBV during latency, has a similar effect on cell growth as K1 encoded by KSHV during lytic replication; LMP2A has a similar function as K15 on inhibition of BCR activation; and the viral cytokines vIL10 and vIL6 encoded individually by EBV and KSHV promote B-cell proliferation. These phenomena support the notion that maintenance of viral latency with intermittent periods of productive viral replication is very common. Thus, the manipulation of the host cell membrane by viral proteins probably contributes to the progression and development of neoplastic diseases associated with EBV or KSHV infection.
In this review, we have discussed the composition and functions of host cell membrane proteins including homologs of receptors and cytokines. These viral proteins elicit many of the same phenotype as their cellular counterparts and therefore provide biological function in virus-infected cell types that do not normally express the corresponding cellular proteins. Our current knowledge about the hijacking of host cell membrane proteins and signaling by human γ-herpesviruses illustrates interesting similarities between proteins with apparently divergent functions at different stages of the viral life cycle. Despite similarities in the signaling pathways engaged by both EBV and KSHV, it is still very hard to answer why the same signaling pathway may result in transformation when targeted by some viral proteins but not others. Expression of membrane receptors, cytokines and chemokines during latency and lytic replication appears to be associated with an essential or at least contributory role in viral tumorigenesis, probably through mimicking physiological signals required for the survival of immune B or T cells.
The most interesting property that these host membrane proteins share is their ability to self-oligomerize for constitutive activation. However, it remains largely unclear whether oligomerization of these membrane proteins is caused by endogenous or exogenous ligands. Although it has been shown that no matter self-or ligand-induced oligomerization and interaction with host cellular factors, these viral transform membrane proteins could be adopted and modified cellular pathways for transforming host cells. In addition, both EBV and KSHV have also been demonstrated to manipulate the host cell membrane through deregulating the expression of both chemokines and cytokines or their receptors. For instance, EBV can induce expression of IL-8 and MIP-1 in human neutrophils (Mccoll et al., 1997); KSHV utilizes microRNA to reduce the production of inflammatory-response cytokines (Abend et al., 2012) and dynamically regulate production of IL-6, TNF-α and MIP1α in dendritic cells after KSHV primary infection (Hensler et al., 2009). This strategy is utilized to interact with other molecules that could directly impair the activation of host cell membrane receptors for viral pathogenesis. In conclusion, the functional elucidation of these cell membrane-associated proteins will provide potentially efficient strategies against EBV or KSHV-associated diseases.
-
The authors would like to apologize to the many researchers who have contributed to this area of research but have not been cited in this review due to space limitations. This work is supported by the National Natural Science Foundation of China (81471930, 81402542), the National Basic Research Program of China (973 Program) (2012CB519001), and the National Key Research and Development Program of China (2016YFC1200400). FW is a scholar of Pujiang Talents in Shanghai. QC is a scholar of New Century Excellent Talents in University.
-
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: