HTML
-
Crimean-Congo hemorrhagic fever virus (CCHFV) is a highly pathogenic tick-borne virus that causes severe human disease with a fatality rate of up to 50% (Schwarz et al., 1997; Ergonul, 2006). CCHFV belongs to the family Bunyaviridea, genus Nairovirus, and its genome consists of three segments, i.e., the S segment encoding nucleoprotein (NP), the M segment encoding glycoprotein (G), and the L segment encoding RNA-dependent RNA polymerase (RdRp). CCHFV is widely distributed around the world and has been reported in over 30 countries in Asia, Africa, and Europe (Ergonul, 2006). However, isolation of CCHFV strains is not simple. Although several cell lines, including Vero, BHK-21, and SW-13 cells, can be used for CCHFV isolation from serum samples of patients in the acute stage, these cells are less sensitive than the traditional method of intracranial inoculation of a CCHFV positive sample into newborn mice (Shepherd et al., 1986). In addition, handling CCHFV in cell culture and animals requires a high-level biosafety laboratory, which is not available in many epidemic countries. Thus, the establishment of suitable animal models has been slow, and there is only limited knowledge on the patho genesis of CCHFV, precluding vaccine design and drug development.
Previous phylogenetic analyses have found that the genomes of the sequenced CCHFV strains from different countries were highly diverse (Papa et al., 2002; Hewson et al., 2004; Deyde et al., 2006; Sun et al., 2009; Zhou et al., 2013a). Seven major groups in association with the geographic distributions, Asia 1 and 2, Africa 1-3, and Europe 1 and 2, were designated based on phylogenetic analyses of S segments (Hewson et al., 2004; Anagnostou and Papa, 2009; Zhou et al., 2013a). However, when phylogenetic trees were built based on M and L segments, many discrepancies were found among the groups, suggesting high sequence diversity of CCHFV M and L segments (Anagnostou and Papa, 2009; Zhou et al., 2013a).
In China, CCHF outbreaks have been previously reported in Xinjiang Province (Sun et al., 2009). The first CCHF cases were recorded in 1965, when a CCHFV strain was identified from a patient living in Bachu County (Papa et al., 2002). The most notorious outbreak of CCHF occurred in Bachu County in 1997, during which five of 26 reported cases (19.4%) were fatal (Papa et al., 2002). Hyalomma spp. ticks are known to be the major vectors of CCHFV (Ergonul, 2006). Because H. asiaticum asiaticum ticks are the dominant population in the desert ecosystem of Xinjiang, epidemiological surveys have been carried out to investigate the prevalence of CCHFV in the desert areas, including Tasim Basin and Junggar Basin. H. asiaticum asiaticum ticks in both basins of Xinjiang were found to be CCHFV positive, and CCHFV strains were isolated from inoculation of newborn mice (Sun et al., 2009), suggesting that Xinjiang Province may be an important area representing a valuable CCHFV reservoir in tick vectors and a threat to local residents.
In this study, we isolated and characterized a new CCHFV strain from H. asiaticum asiaticum ticks collected in Xinjiang Province, China. Infection investigations were performed in newborn mice and the complete genome of S, M, and L segments were sequenced and analyzed. The results provided important insights into our understanding of CCHFV infection in newborn mice and the evolutionary relationships of our novel strain with other Chinese strains, highlighting the prevalence of CCHFV in ticks from the region north of Tarim Basin in Xinjiang.
-
Vero E6 cells obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) were maintained in Dulbecco's modified Eagle medium (DMEM; Gibco) with 10% fetal bovine serum (FBS; Gibco). A monoclonal antibody 43E5 against CCHFV nucleoprotein (NP) was generated at the Xinjiang Centers for Disease Control and Prevention (CDC). Newborn BALB/c mice (2 days old) were obtained from the Animal Center of Hubei Provincial CDC and raised in a biosafety level (BSL)-3 laboratory for subsequent inoculation with tick samples.
-
Ticks were collected from four counties locating in the Tarim Basin of Xinjiang (Yuli, Yutian, Luntai and Minfeng Counties) (Figure 1). In total, 2, 150 H. asiaticum asiaticum ticks were identified morphologically under light microscope by a trained technician. Then the ticks were divided into groups according to different geographic distributions (n=50-70 ticks/group).
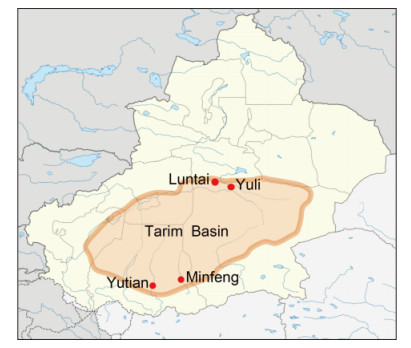
Figure 1. A map of Xinjiang Province showing the locations of the sampling spots for the current study.The red dots indicate the four counties where the tick samples were collected. The light ochre area represents the scope of Tarim Basin in the southern region of Xinjiang Province.
Homogenates of 35 groups diluted in 0.06 mol/L phosphate-buffered saline (PBS, pH 7.4) were centrifuged (3000 rpm, 10 min) to remove cell debris. Supernatants containing 100 U/mL penicillin and 100 μg/mL streptomycin were prepared for inoculation of mice. Newborn BALB/c mice (2 days old) were inoculated with the supernatants by both intracranial (i.c.) and subcutaneous (s.c.) routes (volume: 30 μL). The second passage in mice was performed with brains from some of the sick mice homogenized in 0.06 mol/L PBS. After centrifugation, supernatants were inoculated into other groups of newborn nice. Mouse tissues were collected for further analysis.
Vero E6 cells were incubated with the supernatants from the homogenates of CCHFV-positive mouse brains for 2 h at 37 ℃ with 5% CO 2. The supernatants were then replaced with 5 mL fresh DMEM supplemented with 2% FBS. After 6 days post infection, immunofluorescence assays (IFAs) were performed to detect viral protein expression in cells. Briefly, cells were fixed with 4% paraformaldehyde diluted in PBS and permeabilized by 0.2% Triton X-100. After blocking with PBS containing 5% bovine serum albumin (BSA), the cells were incubated with monoclonal antibodies against CCHFV NP as the primary antibody and then fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse serum (Abcam, Cambridge, UK) as the secondary antibody. Images were visualized under a fluorescence microscope (ECLIPSE TE2000-S; Nikon, Japan).
-
Total RNA was purified from the homogenates of inoculated mouse brains (100 mg) using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. One microgram of RNA was subjected to cDNA synthesis with random primers using M-MLV (Invitrogen) according to the manufacturer's instructions. RTPCR was then performed using the cDNA as a template to detect the CCHFV S segment, as previously described (Rodriguez et al., 1997).
Luminex high-throughput fluorescence fast assays were then performed to detect CCHFV RNA in mouse tissues. RT-PCR was performed in a reaction volume of 25 μL (Qiagen One Step RT-PCR Kit) containing 300-900 ng total RNA purified from each tissue and 5 nmol/L degenerate primers specific for the CCHFV L segment (CCH FV-L-F: 5′-TCAGGYTACTTCTCWGCCAAATG-3′; and CCHF-L-R: 5′-CAGTTTGAGAGGTTRACWGCA ACY-3′). RT-PCR was carried out using the following protocol: reverse transcription at 50 ℃ for 30 min; denaturation at 95 ℃ for 15 min; five cycles of 15 s at 94 ℃, 30 s at 59 ℃, and 30 s at 72 ℃; and another 25 cycles of 15 s at 94 ℃, 30 s at 57 ℃, and 30 s at 72 ℃. The second round of PCR was carried out with biotin-conjugated primers using 30 cycles of 15 s at 94 ℃, 30 s at 56 ℃, and 30 s at 72 ℃. The second round of PCR was followed by hybridization of 5 μL of PCR product with a specific probe coupled to fluorescent beads (5 min at 95 ℃ and 15 min at 56 ℃) and incubation with 10 μL of 1× Streptavidin-R-phycoerythrin (5 min at 56 ℃). The median fluorescence intensity (MFI) of each sample was determined using Luminex 100. Samples for which MFI values were over 200 and were 2 times higher than that of the blank control were considered positive.
-
Tissues from sacrificed newborn mice were fixed in 4% paraformaldehyde diluted in PBS for 24 h. The tissue blocks were then dehydrated with increasing concentrations of ethanol and embedded in paraffin. Serial sections (4 μm thick) were prepared for hematoxylin and eosin (H & E) staining and immunohistochemical (IHC) analysis, and H&E staining and IHC were performed as previously described (Xing et al., 2016). The monoclonal antibody against CCHFV NP was used as the primary antibody to detect NP expression in tissue sections. Nonimmune serum from mice was used as the negative control.
-
In total, 34 primer sets were used to sequence the fulllength genome of the new CCHFV isolate, including 18 pairs of previously reported primers (Zhou et al., 2013b) and another 16 pairs of newly designed primers (Supplementary Table S1). The complete sequences of S, M, and L segments were deposited in GenBank (accession numbers: KY354080, KY354081, and KY354082, respectively). Sequence alignments based on CCHFV S, M, and L segments were performed using CLUSTAL W. Phylogenetic trees were constructed using Mega 6.0 by the method of maximum likelihood (ML) and tested with 1000 bootstrap replicates (Tamura et al., 2013). The segments used for the phylogenetic analysis in this study were the full-length sequences deposited in GenBank.
Cells, antibodies, and mice
Tick collection and virus isolation
Reverse transcription polymerase chain reaction (RT-PCR) and high-throughput fluorescence fast assays
Histopathology and immunohistochemistry of tissues from newborn mice
Complete genome sequencing and phylogenetic analysis
-
Thirty-five groups of newborn mice were inoculated with supernatants clarified from the homogenates of ticks. After the second passage in mice, 11 groups began to demonstrate illness from 4-5 days post inoculation (d p.i.). The signs of illness included slow growth, lethargy, tremors, loss of balance, paralysis of the hind limbs, and progressively diminishing mobility. These mice were sacrificed, and homogenates of brain, heart, liver, spleen, lung, and kidney were prepared. RT-PCR followed by Sanger sequencing showed that the group inoculated with tick homogenates from Yuli County was positive for CCHFV S segment (data not shown). Further, results of high-throughput fluorescence fast assay revealed very high virus load in brain, heart, liver, and kidney (Table 1). BLASTN analysis showed that the sequences of the PCR products shared 99% nucleotide similarity with other CCHFV sequences deposited in GenBank. The supernatants of the homogenates from CCH FV-positive brains were incubated with Vero E6 cells. Green fluorescence was detected in the cytoplasm of Vero E6 cells using IFA (Figure. 2A, white arrowheads), suggesting that Vero E6 cells were infected with CCHFV. Thus, a new strain of CCHFV, designated YL16070, was identified and isolated from tick samples from Xinjiang, and Vero E6 cells were sensitive to the virus after inoculation in mice.
Tissues* MFI Brain* 1698 Heart* 1621.5 Liver* 1613.5 Spleen* 848.5 Lung* 1261.5 Kidney* 1692 Brain from the healthy mouse* 3 CCHFV 1369 Blank 38 Note: *Tissues were collected from newborn mice sacrificed on 5 d p.i. MFI, median fluorescence intensity of each sample. Table 1. MFI values of the tissues from newborn mice
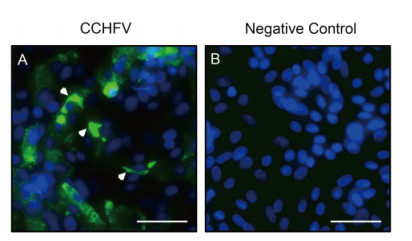
Figure 2. Immunofluorescence detection of CCHFV NP expression in Vero E6 cells. (A) Vero E6 cells were incubated with supernatants from the homogenates of CCHFV-positive mouse brains. At 6 days post infection, CCHFV NP expression was detected by IFA, as indicated by white arrowheads. (B) Healthy cells were used as a negative control. Bars, 1 mm.
-
On 5 d p.i., tissues including the brain, heart, spleen, liver, lungs, and kidneys were all collected, and CCHFV RNA was detected in these tissues (Table 1). Notably, higher levels were found in the brain, heart, liver, and kidneys than in the other tissues (Table 1). Additionally, tissue lesions were noted in pathological sections of the liver, kidneys, and brain (Figure 3A). However, no significant changes were observed in the heart, lungs, and spleen (data not shown). The primary lesions in the liver consisted of ballooning degeneration of hepatocytes, congestion, steatosis, and focal necrosis; infiltration of inflammatory cells was also observed in the liver. The kidneys showed edema and cytoplasmic swelling of poorly stained renal tubular epithelial cells in the renal tubular cortex. Hemorrhagic focuses were found in the brain. IHC staining also found CCHFV infection and antigen expression in the liver, kidneys, and brain on 5 d p.i. (Figure 3B). These results suggested that the liver, kidneys, and brain were the primary replication sites of CCHFV in newborn mice.
Figure 3. Detection of CCHFV infection in newborn mice. (A) Tissues from newborn mice were sectioned and stained, and tissue lesions caused by CCHFV infection were detected. Red arrowheads indicate the lesions. (B) Immunohistochemical analysis of CCHFV antigens in different tissues of newborn mice. Red arrowheads indicate the foci of CCHFV antigens.
-
The genome of the new isolate, designated YL16070, consisted of three segments, the S (1673 nt), M (5379 nt), and L (12156 nt) segments. BLASTN comparisons showed that YL16070 was the most closely related and shared 98% identity with another strain, YL04057, which was isolated from H. asiaticum asiaticum ticks in Yuli County in 2004 (Zhou et al., 2013b). Because the groups of CCHFV were designated according to their geographic distributions, phylogenetic trees built based on 101 complete sequences of S segments showed that eight groups were clustered, i.e., Asia 1 and 2, Africa 1-3, and Europe 1-3, and that YL16070 clustered with all other Chinese strains, belonging to the Asia 2 group (Figure 4A). In the M and L trees, discrepancies were noted, consistent with other studies (Figure 4B and 4C) (Hewson et al., 2004; Anagnostou and Papa, 2009; Chen, 2013; Zhou et al., 2013a). Composed of 90 sequences, the M tree was divided into seven groups, including Asia 1-3, Africa 1-3, and Europe 1 and 2 (Figure 4B); and the L tree (68 sequences included) was divided into six groups, including Asia 1/2 (a group of strains in combination of strains from Asia 1 and 2 in the S and M trees) and 3, Africa 2 and 3, and Europe 1 and 2 (Figure 4C). The Chinese strains did not cluster in just one group in the M and L trees, but dispersed in three Asian groups in the M tree and two Asian groups in the L tree. The new isolate YL16070 belonged to Asia 3 in both the M and L trees. Since there were fewer full-length sequences of M and L segments available for phylogenetic analysis, it is possible that fewer groups were found in the M and L trees than in the S tree. However, the nonexistent group Asia 3 in the S tree appeared in the M and L trees and was composed of at least three Chinese strains. These findings suggested that YL16070 and the closely related Chinese strains (YL04057, 7001, 79121M18, and 79121) may represent a new genotype of CCHFV and that more than one genotype of CCHFV circulated in Xinjiang Province.

Figure 4. Phylogenetic analysis of CCHFV with available complete genome sequences. ML trees were constructed based on the complete sequences of S (A), M (B), and L (C) segments deposited in GenBank. Strains are displayed in the format of "GenBank accession number_strain_country_year". The Chinese strains are shown in red bold characters, and the new isolate in this study, YL16070, is labeled by a black solid circle (●).
Isolation and identification of a new strain of CCHFV from the H. asiaticum asiaticum ticks in Xinjiang
Detection of CCHFV infection in newborn mice
Phylogenetic analysis with the new strain indicated that more than one genotype of CCHFV circulated in Xinjiang Province for years
-
According to previous epidemiological investigations in ticks, CCHFV is widely distributed in Xinjiang (Yen et al., 1985; Feng, 1991; Dai et al., 2006; Sun et al., 2009), and Tarim Basin is considered a natural epidemic focus of CCHF (Sun et al., 2009). For example, Bachu County and the surrounding regions located to the west of Tarim Basin are important areas in which CCHF cases have been reported in the past (Tang et al., 2005; Sun et al., 2009). In this study, we focused on regions to the north and south of Tarim Basin. Tick samples were collected from four counties, i.e., Luntai and Yuli in the northern region and Yutian and Minfeng in the southern region, which were all located in the desert ecosystem of Tarim Basin. A new CCHFV strain, YL16070, was isolated from a group of ticks from Yuli County in this study. In 2004, seven CCHFV strains were isolated from ticks (H. asiaticum asiaticum) collected in Yuli County (Sun et al., 2009; Zhou et al., 2013b). Thus, the isolation of a new CCHFV strain revealed the existence of CCHFV in ticks in Tarim Basin after more than 10 years, and this strain represents a direct threat to local residents.
To date, our understanding of CCHFV pathogenesis is limited. There is a lack of suitable animal models of CCHFV infection, and high BSL laboratories are required for the handling of this virus. Studies have shown that CCHFV infection can induce fatal outcomes in newborn mice and type I interferon receptor-knockout (IFNAR-/-) mice (Gonzalez et al., 1995; Bereczky et al., 2010). High levels of CCHFV RNA were detected in the livers and spleens of IFNAR-/- mice (Bereczky et al., 2010); however, few studies have performed pathological observations of mouse tissues. In this study, we explored CCHFV infection in newborn mice and found tissue lesions and viral antigen expression in the liver, kidneys, and brain. Luminex analysis also found high viral loads in the liver, kidneys, and brain. These results suggested that the liver, kidneys, and brain were replication tissues for CCHFV, improving our understanding of the pathology of CCHFV infection in newborn mice.
Phylogenetic studies have suggested that CCHFV exhibits high genome diversity as a tick-borne virus (Papa et al., 2002; Deyde et al., 2006; Anagnostou and Papa, 2009; Zhou et al., 2013a). Reassortment events may have been involved in CCHFV evolution as many discrepancies were found in clustering of CCHFV strains (Anagnostou and Papa, 2009; Zhou et al., 2013a). We analyzed the phylogenetic relationships of YL16070 compared with other Chinese strains for which fulllength sequences were available. Notably, all Chinese strains clustered in the Asia 1 group in the S tree, but dispersed in different Asian groups in the M and L trees. Additionally, YL16070 clustered with several other Chinese strains and formed a new group in the M and L trees (Asia 3), which represented a different genotype of CCHFV. Furthermore, the Chinese strains could be divided into three Asian groups (Asia 1-3) according to the analysis of M segments, which indicated that more than one genotype of CCHFV had been circulating in Xinjiang Province for years.
In summary, a new strain of CCHFV (YL16070) was isolated from H. asiaticum asiaticum ticks collected in Yuli County located in the Tasim Basin of Xinjiang Province, China. The above analyses further confirmed the existence of CCHFV in ticks in Xinjiang. Because CCHFV may become a risk factor for severe disease outbreaks and epidemics in Xinjiang, careful control of ticks is needed in order to prevent and control CCHF disease outbreaks.
-
This work was supported by the Science and Technology Basic Work Program (2013FY113500) from the Ministry of Science and Technology of China.
-
The authors declare that they have no conflict of interest. Animal experiments in this study were approved by the ethics committees of Wuhan Institute of Virology, Chinese Academy of Sciences (Approval number: WIVH01201 501).
-
RG, JMS, JLL, ZYS, and QGW performed the ticks collection in Xinjiang and prepared the homogenates for newborn mice inoculation; RG and YFZ performed the mice inoculation and harvested the tissue samples, and performed the virus passage and IFA on cells; DL conducted the tissue sections from sacrificed mice and pathology analysis of CCHFV; ZYS and JY performed the CCHFV RNA detection in mice tissues. JMS and SS performed sequence of the genome with the phylogenetic analysis. YJZ and FD conceived of the study; RG and SS wrote the manuscript; YJZ and FD checked and finalized the manuscript.
Supplementary Table S1 is available on the websites of Virologica Sinica: www.virosin.org; link.springer.com/journal/12250.
-
Primers Sequences (5'-3') Locations of primers S segment S1F* TCTCAAAGAAACACGTGCCGC 1-21 S1R* GACAAATTCCCTGCACCA 653-670 S2F* CCCAGTGAGCCGTGAACAT 628-646 S2R* CAGGGTGCATGTAGATCCTG 1168-1187 S3F* CTTTATGAGCTCTTTGCTGATG 1130-1151 S3R* TCTCAAAGATATCGTTGCCGC 1652-1672 M segment M1F* TCTCAAAGAAATACTTGCGGCAC 1-22 M1R* ATGRTGTGYAYYYTGTGGTGT 504-524 M2F* GTGCATGCAAGCCCATCACC 504-523 M2R* CRTTGATCARCTGMARYCCTG 1201-1221 M3 F TTACTATGCCAAAGGGTCTC 1151-1170 M3 R ATTTGACTGGGATGCTATCT 1901-1920 M4F* TWYAAGCTDTGCGAGAACAGTGC 1833-1855 M4R* CTNACAACCCARGGDATTCTTTC 2481-2503 M5F* CGGARGGARAARGTRGAAGAAACY 2439-2462 M5R* GTWGGTTTGAAGGTTGAYTGRACAT 3307-3331 M6F-2‡* CGTGGGGAGCAATCAATGTTC 3316-3336 M6R-2‡* GTGTGCAGCTCTGTAGCTTGC 4030-4050 M7F* GAAGGTTTYTTTGAYYTRATGCATG 3951-3975 M7R* GCTCTGTGGWTGSTCAAGYTT 4719-4739 M8F* AGCTGCASYRAAGAWGAYACACAA 4680-4703 M8R* TCTCAAAGATATAGTGGCGGCAC 5345-5367 L segment L1 F TCTCAAAGATATCAATCCCC 1-20 L1 R GAGTGCTAATGCAAGGTCTT 652-671 L2F* CTCTCAGAATACTGCCACAG 538-557 L2R* GTTGATAACATGACGCCAAGTG 1123-1144 L3F* CCCTGGAACAGGAATAGAAAG 1052-1072 L3R* ATTGCCCTGCCTGAGGTAC 1666-1684 L4-F1 ACTTCTCGGCTTATCACATT 1500-1519 L4-R1 ATTCAACGGAGTCTCAGGTA 2287-2306 L5 F CAAACCAAGTCTGACCATGT 2048-2067 L5 R TCTGTCCTTATTTTCCCGTC 2571-2590 L6F* GACTATGGAGAGAGGGGAATAG 2463-2484 L6R* TGACTTGATGTCTTCACTTAACC 3130-3152 L7 F CTACCCGTTAGTTTGGAACA 2934-2953 L7 R CTATCTTGGGACACCTCTTG 3311-3330 L8 F GATTGAGCTGCTTGCTTATA 3218-3237 L8 R GACCTTGGCACAGAACACTT 3723-3742 L9 F CAACCTATGCAAAAGGAGTA 3661-3680 L9 R TATGATGAGCCAAGGACAGC 4287-4306 L10+ F AGTAAAGGTAAGGCATTGTG 4154-4173 L10+ R GTTTATTCTGTTGTTGGGAG 4616-4635 L11+ F ACCTAGTTTATGGCTTCCTT 4573-4592 L11+ R ACTGGCAGATGTCTGTTATT 5309-5328 L10F* TGCCCAAATGTGAGAAAAGC 5076-5095 L10R* GAGYYTCTTCTTYAAGCTTCC 5889-5909 L11F* CAATCTCTTCHTCAGTYAAAGG 5869-5890 L11R* CTATCTTTGTYTGATGTAGYAGC 6614-6636 L12F* GAYYTRCACAARACCACTGACG 6588-6609 L12R* CTGACCCATATGGTTGTARCTGTT 7473-7496 L13 F TAGACAGTTGGGAAGGAAAT 7396-7415 L13 R TAAAGGCAGTGTTGGTGATA 7955-7974 L14 F CTTATCACCAACACTGCCTTTA 7953-7974 L14 R AAACTCCCAACCTTTCCAAC 8699-8718 L15 F CATTTGGAGGAGAAGGAACT 8587-8606 L15 R AAGGCTCTTTGACAGTGGTAT 9106-9126 L16+ F GATGGTCCACCAAGCACAGC 8980-8999 L16+ R TGCCCTTTAGCATGTAGAAA 9656-9675 L17 F TACAACAAGCAAAGCCAACC 9628-9647 L17 R GTATGTGAACTTCCCAGACC 10129-10148 L16F* CTGTRAAACGAGATTCTGAACGC 10000-10022 L16R* ACCTCCTGATTTGAGGTACTTGTG 10794-10817 L18+ F AGACTACATACCACGAGAATC 10564-10584 L18+ R CTCTGGAAATAGAAGGCACA 11277-11296 L19 F GTTAATGAACTTAATCAGCCAGTG 11183-11206 L19 R ACCCGTCAACATCAGAATCAAATC 11718-11741 L18F* CAAAGCCTGARAGAGTGGTYATRG 11473-11496 L18R* TCTCAAAGAAATCGTTCCCCCCAC 12133-12156 Note: *Primers were used in reference to a previous study of CCHFV genome announcement (Zhou et al., 2013b). Table S1. Primers used to sequence the complete genome of the CCHFV isolate
















 DownLoad:
DownLoad: