HTML
-
Kaposi’s sarcoma-associated herpesvirus (KSHV), also known as Human herpesvirus 8 (HHV8), a member of the gamma herpesvirus family, is the infectious etiological agent associated with all forms of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL) and multicentric Castleman’s disease (Razonable, 2011; Zhang et al., 2017). Since its first discovery in 1994(Chang et al., 1994), KSHV has been well studied worldwide (Schwartz et al., 2008; Bagni and Whitby, 2009). Unlike most herpes viruses, KSHV infection is not ubiquitous in the general population. In Asia, North America and West Europe, the prevalence is about 1–10% (Zhang et al., 2014b; Ahmadi Ghezeldasht et al., 2015). However, previous studies have demonstrated that KSHV was endemic in parts of Africa and Mediterranean region, with the prevalence varied from 30% to 50% above (Whitby et al., 1998; Biryahwaho et al., 2010). These studies were of indicative disparities of KSHV prevalence among different geographical regions. Moreover, it has been well documented that HIV was the most important factor associated with KSHV infection (Zhang et al., 2012; Rohner et al., 2016; Liu et al., 2017b), especially in the men who have sex with men (MSM), among whom KSHV has been extensively investigated and some convincible results were obtained. However, among intravenous drug users (IDUs), a population at high risk of HIV infection, results of KSHV infection derived from previous studies were quite diverse. Atkinson et al (Atkinson et al., 2003) reported that KSHV prevalence was 10% in men who practicing intravenous drug use, which was much lower than our previous result (Zhang et al., 2014a). Undoubtedly, the considerable variability of KSHV infection among IDUs (Simpson et al., 1996; Rezza et al., 1998; Wang et al., 2000; Lee et al., 2014) hampered the accurate understanding of KSHV epidemiology in this special population.
Therefore, we, for the first time, performed a comprehensive meta-analysis to obtain the global prevalence of KSHV among IDUs. These results will accelerate the completion of picture depicting the epidemiology of KSHV among IDUs, and assist the better understanding of KSHV transmission route.
-
We conducted a comprehensive search of literature in Medline (PubMed), EMBASE, Web of Science, the Chinese Biomedical Literature Database (CBM), the China National Knowledge Infrastructure Database (CNKI) and Wanfang Database. The search strategy was listed in Supplementary Table S1. The references of reviews were also examined to search for additional eligible studies.
-
Studies were eligible if they fulfilled the following criteria: 1) reporting data on prevalence of KSHV infection (a positive result for antigens or virus DNA) among IDUs (drug users who ever had an injecting history) from any region in the world; 2) testing KSHV antibodies or DNA for virus detection; 3) cohort studies, case-control studies, cross-sectional surveys, or randomized controlled trials documenting the prevalence of KSHV infection with a minimum sample size of 50 participants in total. We excluded publications that were: 1) of which study population covered by other articles from the same research group; 2) non-original studies (including commentaries, reviews, meta-analyses, correspondence, editorials); 3) lacking of sufficient information.
The titles retrieved from this research were reviewed independently by two researchers (QF and ZL). The third researcher was involved in the decision-making in case of any disagreement. At least three authors (including first author) further reviewed the abstracts and full texts of the included papers before making a final decision on which studies to be included in this meta-analysis. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement was followed as a guideline for conducting this systematic review and meta-analysis.
-
The following information was extracted from each study: first author, year of publication, study location (country, continent), testing method of KSHV, age range and gender distribution of the participants, number of KSHV-positive cases and sample size of the IDUs (total and stratified by HIV-infected status). If any data essential to the analysis were not available for a study, best efforts were made to contact the authors to obtain the missing data. Two researchers extracted the information independently. Inconsistencies were resolved by examining the original paper and discussing the discrepancy until a consensus was reached.
-
The number of KSHV infected cases and all participants in each study were combined to obtain a pooled proportion and 95% confidence interval (CI). Subgroup analysis was conducted to aggregate the prevalence among IDUs with different HIV-infection status and from different continents. The overall prevalence was presented via forest plots. Considering the variance stabilization, 0.5 was added to event number and sample size of studies with an event probability of either 0 or 1 and log-transformation of the original prevalence was implemented to calculate an overall prevalence according to the Shapiro-Wilk normality test (Luo et al., 2013). Heterogeneity across the included studies was tested using the Q-test, and the results were considered statistically significant when P < 0.1. I2 metric was also used to quantify heterogeneity. When the effects were assumed to be homogeneous (P > 0.1, I2 < 25%), the fixed-effects model was used; otherwise, the random-effects model was applied. Between-study heterogeneity, if existed, was further explored using meta-regression analysis, in which we evaluated the effect of variables included in the data extraction form. Dummy variables were created for the categorical variables. Publication bias was examined with funnels plots, which showed a scatter plot of studies included in the meta-analysis. An asymmetrical appearance of dots in the funnel plots can be regarded as a proof of publication bias. Egger weighted regression methods (Egger’s test) were used to statistically assess publication bias. All analyses were performed using R package Meta (Version 4.8-2) and Metafor (version 2.0-0). A two tailed P < 0.05 was considered statistically significant, unless otherwise specified.
-
A total of 193 abstracts were retrieved after duplicates were removed. Through preliminary screening of their titles and abstracts, we excluded 149 studies due to irrelevance and undesirable study design. After further full-text reading, additional 22 studies were excluded. A full PRISMA record management flow chart was presented in Figure 1. Finally, twenty-two studies were eligible for this meta-analysis according to the inclusion and exclusion criteria. A total of 7881 IDUs, involving 3611 HIV-uninfected and 2504 HIV-infected individuals, were included (Table 1). Variables, such as age and gender, were not shown in Table 1 or subsequently analyzed in the meta-regression due to the scarcity of original data.
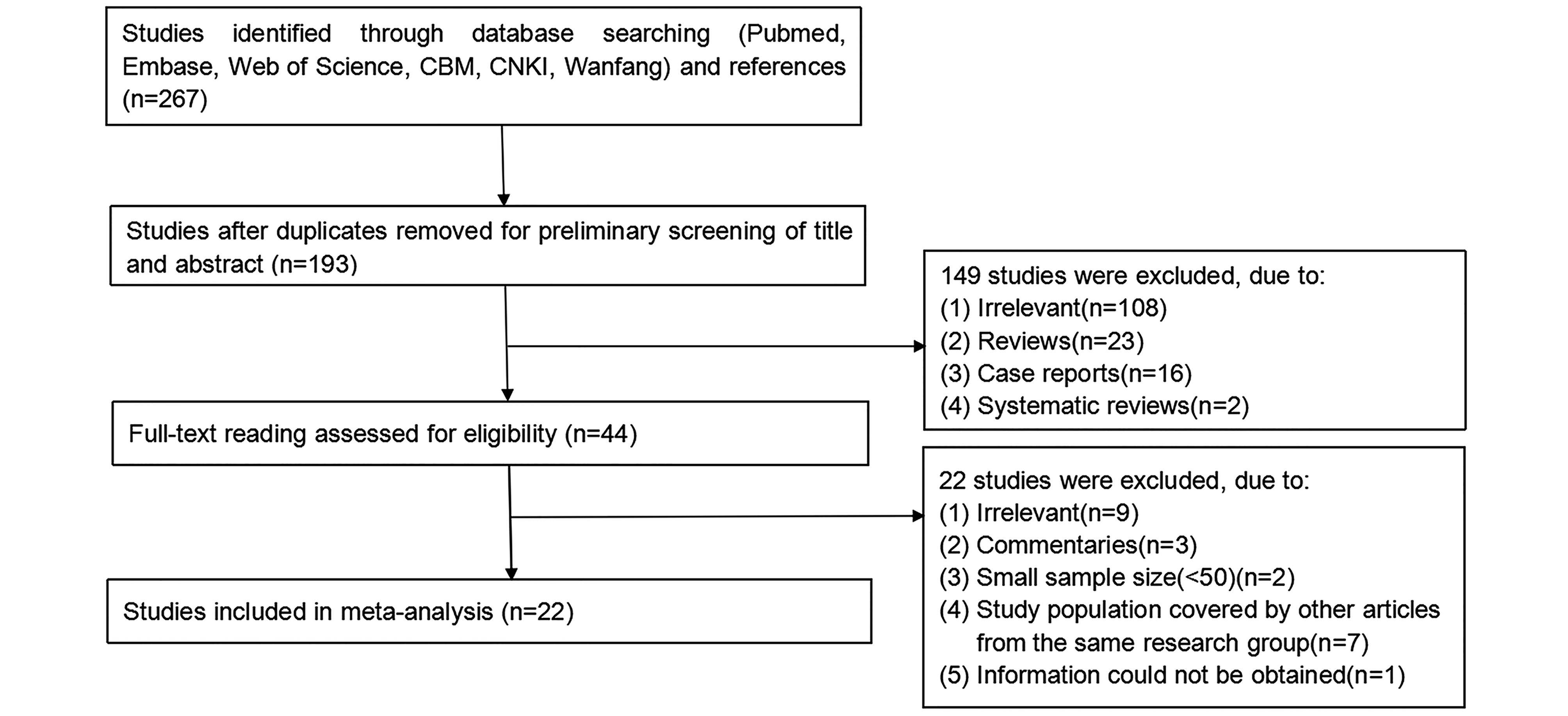
Figure 1. Flow chart of the study selection process and specific reasons for exclusion from meta-analysis.
Authors Year Continent(Country) Testing Method KSHV positivity (No. of positive sera/No. of tested sera) Total HIV-positive HIV-negative Khajedaluee et al. 2016 Asia (Iran) ELISA 8/111 – – Kakavand-Ghalehnoei et al. 2016 Asia (Iran) PCR 8/60 – – Lee et al. 2014 Asia (China) ELISA 27/553 25/377 2/176 Zhang et al. 2014 Asia (China) IFA 56/296 – – Zavitsanou et al. 2010 Europe (Greece) ELISA 70/288 – 67/286 Yang et al. 2010 Asia (China) ELISA 58/203 – – Larocca et al. 2005 Europe (Italy) IFA 14/134 8/32 6/102 Bernstein et al. 2003 America (US) IFA 45/390 38/294 7/96 Atkinson et al. 2003 America (US) ELISA 218/1905 55/233 163/1672 Goedert et al. 2003 America (US) ELISA 14/137 14/137 – Parisi et al. 2002 Europe (Italy) IFA 13/155 13/155 – 2002 Europe (Italy) IFA 18/85 18/85 – Sosa et al. 2001 America (Argentina) IFA 26/153 25/144 1/9 Greenblatt et al. 2001 America (US) IFA 40/237 35/178 5/59 Gambus et al. 2001 Europe (Spain) IFA 44/382 29/254 15/128 Cannon et al. 2001 America (US) ELISA 141/771 – – Diamond et al. 2001 America (US) IFA 9/65 – – Perna et al. 2000 Europe (Italy) IFA 62/374 32/163 30/211 Wang et al. 2000 Asia (China) ELISA 63/107 – – Rezza et al. 1999 Europe (Italy) IFA 20/133 20/133 – Renwick et al. 1998 Europe (The Netherlands) ELISA 89/1167 26/351 63/816 Rezza et al. 1998 Europe (Italy) IFA 55/112 26/47 29/65 Simpson et al. 1996 Europe (UK) ELISA 2/63 2/38 0/25 Table 1. The detailed characteristics of all eligible studies
-
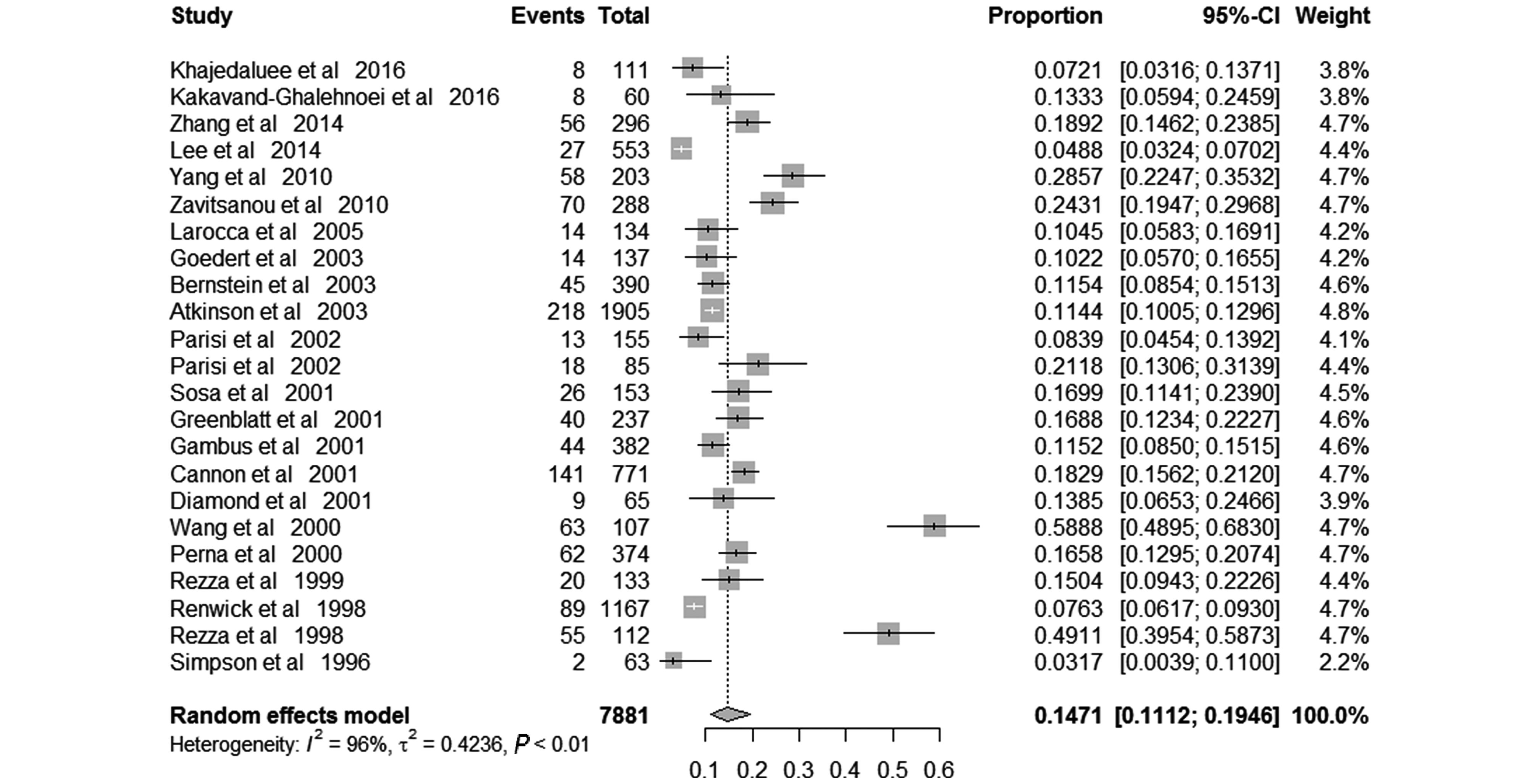
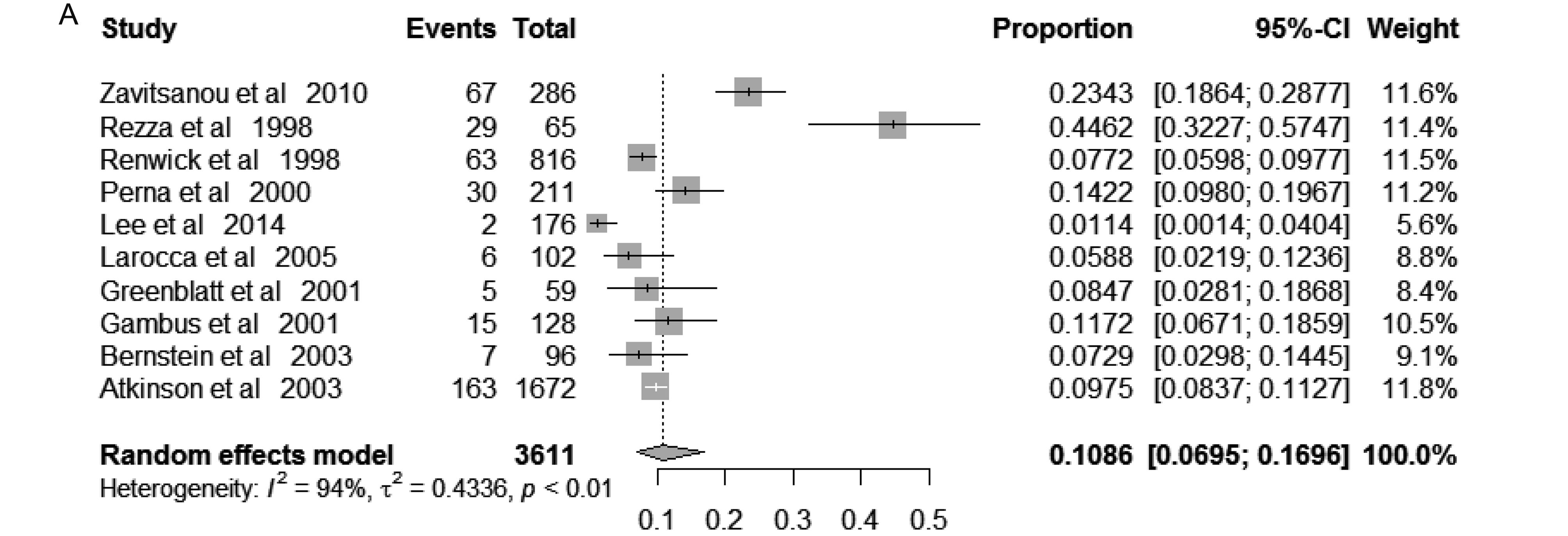
The pooled prevalence of KSHV among IDUs was 14.71% (95% CI 11.12%– 19.46%), varied from 3.17% to 58.88% across included studies. Random-effects model was applied because of the significant between-study heterogeneity (I2 = 96%; P < 0.01) (Figure 2). In those free of HIV, the pooled KSHV prevalence was 10.86% (95% CI, 6.95%–16.96%; I2 = 94%; P < 0.01), varied from1.14% to 44.62% (Supplementary Figure S1a), while in those infected HIV, the pooled KSHV prevalence was 13.56% (95% CI, 10.57%–17.38%; I2 = 83%; P < 0.01), varied from 6.63% to 23.61% (Supplementary Figure S1b).
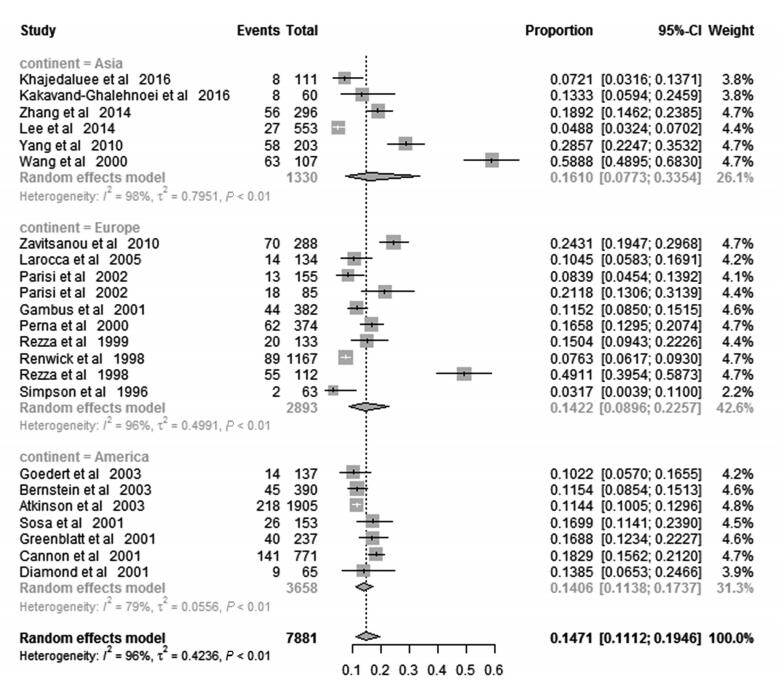
To explore geographical diversity of KSHV distribution, the variability across different regions was assessed as well (Figure 3). Overall, the pooled KSHV prevalence among IDUs in the three continents involved in the present meta-analysis was 16.10% (95%CI 7.73%–33.54%) in Asia, 14.22% (95%CI 8.96%–22.57%) in Europe, 18.24% (95%CI 11.63%–28.61%) in the Mediterranean part and 14.06% (95%CI 11.38%–17.37%) in America, respectively. There is no significant difference in the KSHV prevalence amongst these geographical regions.
-
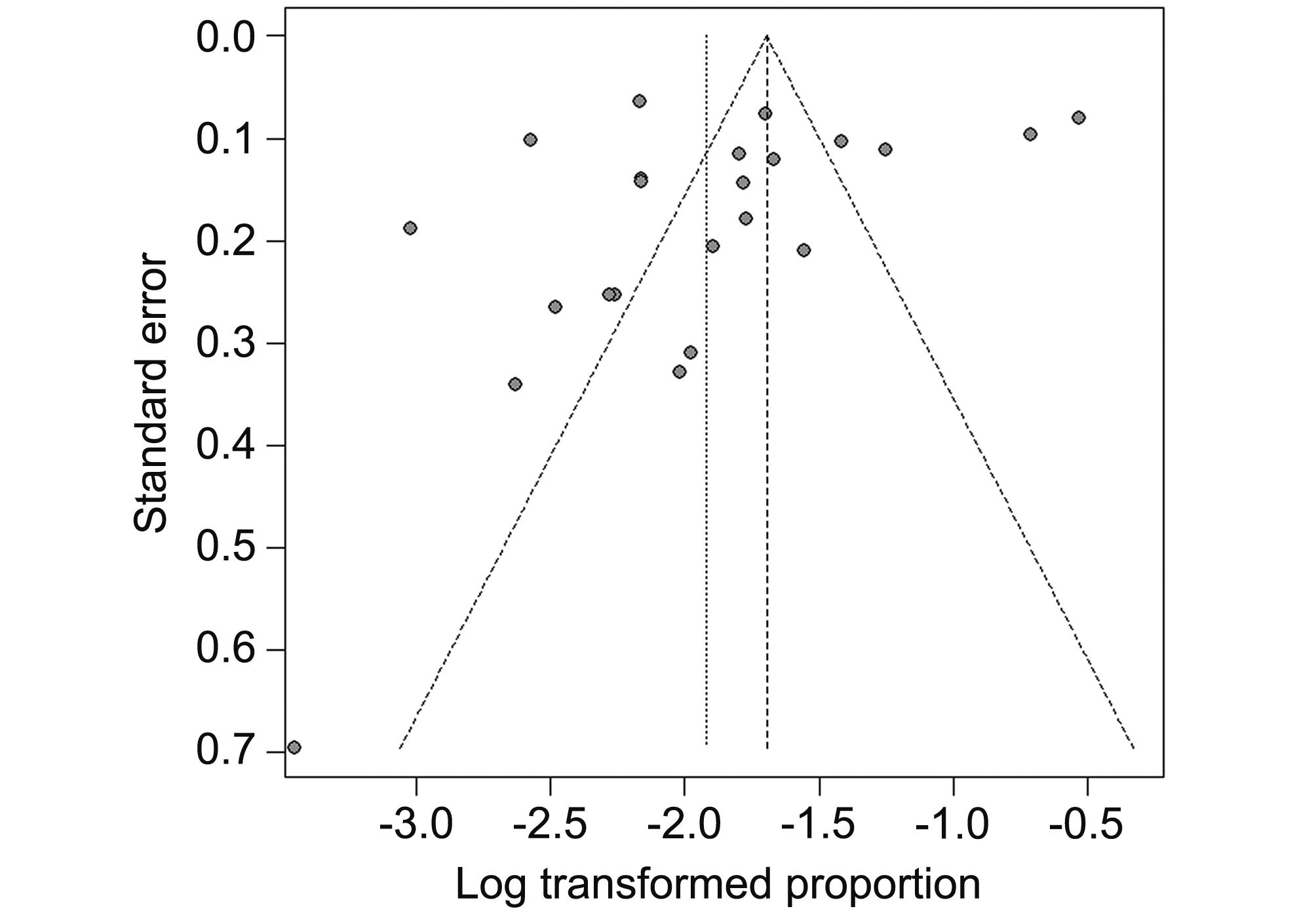
No publication bias was detected in terms of Egger’s test (t = –1.37, P = 0.18), and the shape of the funnel plots did not reveal any evidence of asymmetry (Figure 4).
The variables including publication year, study regions and testing assays were fitted in the multivariate model of meta-regression. None of the three variables proved to be statistically significant (Table 2).
Standard error Z value P value Regression coefficient (95%CI) Constant 73.62 1.55 0.12 113.88 (–30.40, 258.17) Year 0.04 –1.58 0.12 –0.06 (–0.13, 0.01) Continent America (reference) – – – – Asia 0.51 1.57 0.12 0.79 (–0.20, 1.79) Europe 0.33 –0.12 0.91 –0.04 (–0.68, 0.60) Testing method ELISA (reference) – – – – IFA 0.31 0.82 0.41 0.25 (–0.35, 0.86) PCR 0.79 0.16 0.88 0.12 (–1.43, 1.67) Table 2. Meta-regression analysis showing influence of variables on the heterogeneity of prevalence (N = 7881)
-
In the present meta-analysis, we found a moderately higher KSHV prevalence (14.71%, 95% CI, 11.12%–19.46%) among IDUs in contrast to the general population (Moore, 2000) and no significant difference was detected among subgroups, thereby highlighting the vulnerability of KSHV infection in IDUs regardless of the HIV status and geographical region.
A previous systematic review reported that the KSHV seroprevalence was 47% in HIV-positive population and 24% in HIV-negative population which suggested an increased risk of KSHV seropositivity was associated with HIV-infection (Rohner et al., 2016). In the current study, higher KSHV prevalence was found in HIV positive IDUs than those free of HIV, albeit the difference was not statistically significant. This result might partly be explained by the between-study heterogeneity and also to some extent imply the absolute risk of intravenous drug use for KSHV infection. However, IDUs seem to have a lower KSHV prevalence when compared with MSM (Liu et al., 2017a). According to our pre-published results, KSHV prevalence was approximately double fold in MSM than that in IDUs. This discrepancy might suggest KSHV is much more likely to be transmitted via sexual behaviors while not blood, though controversy remains (Kedes et al., 1996; Vitale et al., 2000; Minhas and Wood, 2014). Since it is difficult to synthesize information on behavior-associated risk factors quantitatively due to the lack of data, the current meta-analysis could not resolve the perplexity precisely. Further studies focusing on more restricted target population, for example, blood donors, transfusion recipients or children without high-risk sexual practice, are warranted.
Moreover, no significant disparity of KSHV prevalence was detected across geographical regions included in this analysis, while according to previous studies, the KSHV infection were deemed to be low in Asia, Europe except Mediterranean regions, and America (Tornesello et al., 2010; Stiller et al., 2014; Zhang and Wang, 2017). An elevated KSHV prevalence was observed within IDUs compared with general population. In the current study, seven studies were from either Italy or Greece, where were endemic of KSHV(Schwartz, 2004). Nevertheless, the KSHV prevalence in Europe was not significantly higher than that in other regions among IDUs. This result may partly ascribe to the between-study heterogeneity, as regional differences have been documented by Whitby et al. in Italy: a low prevalence was reported for the northern part, whereas a prevalence of 35% was detected in Sicily (Whitby et al., 1998). Moreover, since no publication bias was detected in our study, the shrunken gap of KSHV infection among IDUs from different geographical regions compared with general population may also serve as a reminder of the role intravenous drug abuse played in the transmission of KSHV.
Unfortunately, limited information on the KSHV seroprevalence among IDUs in Africa is available, though comprehensive literature research has been performed. Generally, in Africa, particularly the sub-Saharan part, both HIV and HHV8 are endemic (Butler et al., 2009). Therefore, it is not hard to expect an appallingly high KSHV seroprevalence among IDUs, because injection drug use is increasingly contributing to the HIV epidemic across sub-Saharan Africa (Syvertsen et al., 2015). This fact of missing information on the KSHV prevalence among IDUs in Africa highlights the unmet study for KSHV in Africa and calls for wider range of KSHV investigations.
Some limitations of this study should be mentioned. First, significantly higher between-study heterogeneity was noted. A meta-regression was conducted to identify the potential sources, but no significant variable was detected. Second, data from Africa, South America and Australia were not available, thereby undermining the accuracy and representativeness of overall KSHV prevalence. Finally, some variables such as age and sex have not been evaluated in our study due to the dearth of data. Therefore, the pooled results should be interpreted with caution.
In conclusion, the prevalence of KSHV among IDUs was higher compared to general population, regardless of geographical location and HIV-infected status. To our best knowledge, this is the first systematic analysis on the epidemiologic characteristics of KSHV infection among IDUs. It can be used as an important supplement to the existing KSHV data, elucidate the gaps in the epidemiologic characteristics of this virus, as well as provide evidence for changing KSHV prevention practice in the marginalized population. Given the paucity and some limitations of the available studies, more well-designed researches should be performed in the future to depict the epidemiology of KSHV and to enrich the present findings, especially in Africa.
-
This work was supported by the Natural Science Foundation of Shanghai (17ZR1401400), the Natural Science Foundation of China (grant no. 81772170, U603117) and the Doctoral Fund of Ministry of Education of China (Grant No. 20120071120050).
-
The author declares no conflict of interest. This article does not contain any studies with human or animal subjects performed by the author.
-
QWF, ZQL and TJZ designed the experiments. QWF, ZQL and YZ searched literature, collected and summed up the data. QWF, YZ and ZJZ analyzed the data. QWF, ZQL wrote the paper. All authors read and approved the final manuscript.
Supplementary figures/tables are available on the websites of Virologica Sinica: www.virosin.org; link.springer.com/journal/12250.
-
Database Search strategy Results PubMed (prevalen* OR inciden* OR epidemiolog* OR seroprevalen* OR sero-prevalen* OR seroepidemiolog* OR sero-epidemiolog* OR seropositiv* OR sero-positiv* OR seroepidemiologic studies[MeSH] OR prevalence[MeSH] OR incidence[MeSH]) AND (("Drug Users"[Mesh] AND ("Injections, Intravenous"[Mesh] OR "Substance Abuse, Intravenous"[Mesh])) OR ((intraven* OR inject*) AND drug)) AND (("HHV-8" OR "HHV 8" OR "KSHV" OR "Human Herpesvirus 8" OR "Kaposi's Sarcoma-Associated Herpesvirus" OR "Kaposi's Sarcoma Associated Herpesvirus" OR "Kaposis Sarcoma-Associated Herpesvirus" OR "Kaposi Sarcoma-Associated Herpesvirus" OR "Kaposi Sarcoma Associated Herpesvirus") OR "Herpesvirus 8, Human"[Mesh]) 39 EMBASE 1. exp Human herpesvirus 8/ 2. ("human herpesvirus 8" or "hhv-8" or "hhv8" or "kshv" or "kaposi sarcoma associated herpesvirus" or "kaposi’s sarcoma associated herpesvirus" or "kaposi sarcoma-associated herpesvirus" or "kaposi’s sarcoma-associated herpesvirus" or "kaposi NEAR/3 herpesvirus" or "kaposi NEAR/3 virus").mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 3. exp seroprevalence/ or exp prevalence/ 4. exp seroepidemiology/ 5. exp incidence/ 6. (prevalen* or inciden* or epidemiolog* or sero*epidemiolog* or sero*prevalen* or sero*positiv*).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 7. exp intravenous drug abuse/ 8. (drug and (intraven* or inject*)).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] 9. 1 or 2 10. 3 or 4 or 5 or 6 11. 7 or 8 12. 9 and 10 and 11 162 Web Of Science TOPIC: ("human herpesvirus 8" OR "hhv-8" OR "hhv8" OR "kshv" OR "kaposi sarcoma associated herpesvirus" OR "kaposi’s sarcoma associated herpesvirus" or "kaposi sarcoma-associated herpesvirus" or "kaposi’s sarcoma-associated herpesvirus" or "kaposi NEAR/3 herpesvirus" or "kaposi NEAR/3 virus") AND TOPIC: (prevalen* or inciden* or epidemiolog* or sero*epidemiolog* or sero*prevalen* or sero*positiv*) AND TOPIC: (drug and (intraven* or inject*)) 39 CBM (("herpesvirus 8, human"[weighted: exploded]) OR ("KSHV"[common fields] OR "human herpesvirus 8"[common fields] OR "kaposi sarcoma associated herpesvirus"[common fields] OR "herpesvirus 8, human"[subject terms] OR "HHV-8"[common fields] OR "HHV8"[common fields] OR "herpesvirus 8"[common fields] OR "Kaposi%virus"[common fields]) AND (("Substance Abuse, Intravenous"[unweighted: exploded]) OR ("drug abuse"[common fields]) OR ("drug"[common fields])) (in Chinese) 5 CNKI (SU='KSHV' or SU='HHV-8' or SU='HHV 8' or SU='HHV8' or SU='herpesvirus 8' or SU %'Kapo%virus') and (SU%'drug abuse' or SU%'drug') (in Chinese) 11 Wanfang (Topic:("KSHV or HHV-8 or HHV 8 or herpesvirus 8") + Topic:(Kapo%virus ))* Topic:("drug abuse" or "drug") (in Chinese) 10 Table S1. Search strategy of the databases
















 DownLoad:
DownLoad: