-
Dear Editor,
Zika virus (ZIKV) is a mosquito-borne virus that belongs to the Flavivirus family along with dengue virus (DENV), yellow fever virus, West Nile virus, and Japanese encephalitis virus (Ming et al.2016).ZIKV is a singlestranded positive-sense RNA virus encoding three structural proteins, including nucleocapsid protein C, prM/M, envelope glycoprotein E, and seven non-structural proteins. Since 2015, over 70 countries and territories had reported continuing vector-borne transmission of ZIKV, making it a global public health issue.People infected with ZIKV normally have no or mild symptoms that include fever, rash, muscle pain, red eyes, headache, and conjunctivitis. However, a 2015 survey in Brazil found that the number of microcephaly cases dramatically increased, suggesting that ZIKV infection and newborn deformity were closely related (Heukelbach et al.2016).Additionally, ZIKV infection can cause other severe neurological disorders, such as Guillain-Barré syndrome (Cao-Lormeau et al.2016). Currently, there is no clinically approved vaccine available.
Recently, application of a series of experimental ZIKV vaccines resulted in reduced viral load in several models of animal infection.These included inactivated virus and plasmid DNA-based vaccines (Abbink et al.2016; Larocca et al.2016b), an attenuated live virus based vaccine (Xie et al.2018), a recombinant adenovirus vector-based vaccine (Larocca et al.2016a; Liu et al.2018; Xu et al. 2018), and a liposome nanoparticle-based mRNA vaccine (Pardi et al.2017).Similar to other Flaviviruses, such as DENV, the ZIKV envelope protein E mediates viral binding to cellular receptors and membrane fusion, and it represents the target for most neutralizing antibodies (Lazear and Diamond 2016).The prM protein typically associates with protein E to form a heterodimer and is important for proper folding of protein E.Co-expression of prM and E proteins of several Flaviviruses, including ZIKV, results in secretion of virus-like particles (Boigard et al.2017).In addition, the mRNA vaccine that encodes the full-length prM and E proteins can resist ZIKV infection (Pardi et al.2017; Richner et al.2017).Therefore, prM-E proteins have been the primary targets of most ZIKV vaccine candidates.
Flaviviruses often show antigenic cross-reactivity, which can be beneficial and result in cross-protection. However, humoral cross-reactivity can also exacerbate disease via antibody-dependent enhancement (ADE), of which DENV is the prototypic model (Guzman and Harris 2015).Because ZIKV and DENV share primary vectors (Aedes aegypti and Aedes albopictus for transmission), it is likely that many people infected with ZIKV will be more prone to future infection with DENV serotypes.In such cases, it should be considered whether the presence of a preexisting ZIKV-neutralizing antibody would enhance subsequent DENV infection.This is especially important in vaccine development, because both prM-E and E antigens represent the primary targets of ZIKV-specific neutralizing-antibody induction, which could possibly result in ADE-related effects.Indeed, a previous study showed that a ZIKV-induced antibody response enhanced DENV serotype 2 replication in vitro (Kawiecki and Christofferson 2016).Therefore, to increase safety, it is necessary to investigate other protective ZIKV antigens, besides prM-E or E proteins, for vaccine development.
The capsid protein plays a crucial role in Flaviviridae biology, with a report indicating that a DENV vaccine engineered with a capsid protein alone produced neutralizing-antibody independent immunity and significantly reduced viral loads in the brains of challenged monkeys (Gil et al.2014).In the present study, a recombinant vesicular stomatitis virus (VSV)-based vaccine carrying the ZIKV capsid protein (VSV-Capsid) was generated.VSV has been shown to be an excellent vector to deliver foreign antigens as a viral vaccine vector (Roberts et al.1999). Recombinant VSV has been successfully developed for a number of vaccine candidates, such as human immunodeficiency virus (Rose et al.2001), severe acute respiratory syndrome virus (Kapadia et al.2005), hepatitis B virus (Cobleigh et al.2013), influenza virus (Roberts et al. 1998), papillomavirus (Reuter et al.2002), human respiratory syncytial virus (Kahn et al.2001), Ebola virus and Marburg virus (Jones et al.2005; Geisbert et al.2008).To generate recombinant VSV vaccines, the coding sequence of the ZIKV strain PRVABC59 capsid was amplified by PCR and inserted into VSV backbones between the G-L junction using Nhe I and Xho I restriction enzyme sites (Fig. 1A).Further, these genes were expressed from a single adjacent 30promoter as a distinct transcriptional unit on the G and L genes.The recombinant VSV-ZikaE260- 425 virus expressing the ZIKV E protein DIII domain (E DIII; amino acids 260-425) was used as a parallel control, because ZIKV E DIII is the major antigen region and most specific neutralizing epitopes and E DIII-targeted-antibodies protect mice against lethal infection (Zhao et al. 2016).Recombinant VSVs were recovered through the reverse genetic system in BHK-21 cells, as previously reported (Lawson et al.1995).Cytopathic changes were observed during the viral-packaging process in infected cells (Supplementary Fig. S1A), and viral titers were determined by TCID50 and calculated using the ReedMuench method.We observed that capsid protein insertion resulted in an approximately tenfold attenuation of VSV replication (Supplementary Fig. S1B).N-terminal flagtagged capsid and ZikaE260-425 protein expression was confirmed by western blot at 12-h post-infection using antiFlag and anti-E antibodies (Fig. 1A).Immunoblotting further verified capsid and ZikaE260-425 expression in infected cells (Supplementary Fig. S1C).

Figure 1. Recombinant VSV-ZikaE260-425 and VSV-Capsid vaccines induce immune response and provide specific immune protection in BALB/c mice.A Schematic of the construction of the ZIKV envelope and capsid expression vectors and immunoblot analysis of VSVZikaE260-425 and VSV-Capsid expression in BHK-21 cells infected with recombinant VSVs (10 MOI for 12 h) using ZIKV-envelope and Flag antibodies.GAPDH was used as a loading control.B Anti-ZIKV serum titer was analyzed by ELISA using supernatant from Vero cells infected with recombinant VSV-ZIKV (106 PFU).C Groups of BALB/c mice were vaccinated with 106 PFU VSV-ZikaE260-425 or VSV-Capsid via single intranasal inoculation.Immune responses were detected at the indicated time points.Specific lymphocyte proliferation was detected by BrdU assay in the spleen of mice at 5 weeks post-immunization.D Flow cytometric analysis of antigenspecific IFN-γ release from splenocytes in recombinant VSVimmunized BALB/c mice after in vitro stimulation with inactivated ZIKV PRVABC59.E Groups of BALB/c mice were vaccinated with 106 PFU VSV-ZikaE260-425 or VSV-Capsid via single intranasal inoculation.Five weeks post-immunization, the mice were infected with 104 PFU ZIKV PRVABC59, and the viral loads were measured in the brains, spinal cords, and testes of mice immunized 4 days post infection.The relative viral RNA level was normalized with GAPDH gene and set the level in VSV-GFP immunized mice as 1.Error bars indicate SD.*P < 0.05, **P < 0.01, and ***P < 0.001, Student's t test.
To assess the efficacy of VSV-Capsid-induced antigenspecific antibodies, 6- to 8-week-old male BALB/c mice were randomly divided into four groups and intranasally immunized with 106 PFU VSV-ZikaE260-425 or VSVCapsid, with phosphate-buffered saline (PBS)- or VSVgreen fluorescent protein (GFP)-administered groups used as controls (Supplementary Fig. S2).Antigen-specific antibody immune responses were determined by enzymelinked immunosorbent assay (ELISA) at 1, 3, and 5 weeks post-immunization.Both VSV-Capsid and VSVZikaE260-425 immunization induced high levels of IgG at 3 weeks post-vaccination as compared to control PBS-and VSV-GFP-treated mice.Moreover, higher levels of antigen-specific IgG were observed in VSV-ZikaE260-425- immunized mice relative to VSV-Capsid-immunized mice (Fig. 1B).These results indicated that the VSV-Capsid vaccine was capable of inducing a strong ZIKV-specific humoral response comparable to VSV-ZikaE260-425.
The cellular immune response is also important for host protection during infection.Therefore, we monitored ZIKV-specific T lymphocyte proliferation post-immunization.Splenic lymphocytes from mice at 39 days post-immunization were harvested and seeded in 96-well plates (Corning, NY, USA), followed by stimulation with inactivated ZIKV for 72 h.Significant antigen-specific T cell proliferation was measured by BrdU assay (Roche, Basel, Switzerland), which revealed increased proliferation in VSV-Capsid-and VSV-ZikaE260-425-immunized mice as compared to VSV-GFP-immunized mice (Fig. 1C).To further characterize the cellular responses, we evaluated the ability of splenic lymphocytes to generate interferon (IFN)-γ post-immunization.Splenic lymphocytes were harvested and stimulated with inactive ZIKV for 24 h, followed by treatment with propidium monoazide (PMA), inomysine, and bafilomycin A (Sigma-Aldrich, St.Louis, MO, USA) for 6 h and staining for flow cytometric analysis.VSV-Capsid immunization significantly increased IFN-γ+CD8+, and CD4+T cells in immunized mice relative to VSV-GFP-immunized mice, while VSVZikaE260-425 immunization resulted in lower levels relative to VSV-Capsid-immunized mice (Fig. 1D).
To determine whether VSV-Capsid immunization could protect mice from ZIKV infection, we established a ZIKVinfected mouse model.Mice were intraperitoneally infected with 104 PFU ZIKV PRVABC59, and the E viral gene level was quantified as an indicator of viral replication in the brain, spleen, blood, spinal cord, and testis of infected mice at different time points.Increased levels of viral replication were observed in the spinal cord and brain at 2 and 4 days post-infection, respectively.Viral replication in the testis was modest relative to the spinal cord and brain at 2 or 4 days post-infection (Supplementary Fig. S2).The immunized mice were subsequently challenged with ZIKV, and quantitative PCR was performed at 4 days post-infection to measure viral replication.As shown in Fig. 1E, VSV-Capsid and VSV-Zika E260-425 immunization, respectively, significantly reduced ZIKV-replication loads in the brain and spinal cord as compared with VSV-GFPimmunized mice.Although there was no statistical difference observed in the testis due to the large variation, we observed reduced viral replication in the testis of VSVCapsid-immunized mice compared to VSV-GFP-immunized mice (Fig. 1E).These results indicated that the VSV Capsid vaccine was effective against ZIKV infection following single-dose immunization.
Although the capsid is mainly related to viral replication, studies report that in DENV4, the capsid protein epitope can be recognized by cytotoxic T lymphocytes, making it a target for antiviral T cell responses (Gagnon et al.1996).Additionally, immunization with the capsid alone can induce a protective immune response independent of neutralizing antibodies and primarily dependent upon cell-mediated immunity (Lazo et al.2007).In the present study, we constructed a recombinant VSV-Capsid vaccine, which resulted in a slightly lower number of generated antibodies than VSV-ZikaE260-425 in immunized mice.This might be attributed to structural features of the capsid, which is not exposed on the cell surface (Teoh et al.2012).However, in cellular immune response assays, the VSV-Capsid vaccine resulted in elevated specific T lymphocyte proliferation and IFN-γ secretion relative to the VSV-Zika E260-425 vaccine, indicating its potent ability to induce a cellular immune response. Importantly, the viral load was significantly reduced in the brains and spinal cords of challenged mice, indicating the protective role of the VSV-Capsid vaccine in immunized mice against ZIKV infection.It is possible that the VSVCapsid vaccine also protects fetuses in immunized pregnant mice because less virus can cross the placental barrier to establish fetal infection.Moreover, although a VSVbased vaccine VSV-EBOV has been proven safe in trials in Africa and Europe (Agnandji et al.2016), it is ideal to receive vaccination before pregnancy.
In conclusion, we have developed a novel vaccine expressing ZIKV capsid protein as antigen.Vaccination with VSV-Capsid provided strong immune responses as well as effective protection upon ZIKV challenge in mice. Our findings provide insight into the importance of ZIKV capsid protein for the further development of ZIKVvaccine.
HTML
-
We gratefully thank Dr.Feifei Yin in Hainan Medical University for providing ZIKV PRVABC59 and also like to thank Prof.Yi Shi in Institute of Microbiology, Chinese Academy of Sciences for providing the Zika E monoantibody.This work was supported by the National Natural Science Foundation of China (31470848, 31470880, 31670898, and 31870867), Open Research Fund Program of the State Key Laboratory of Virology of China (2017IOV003) and Jiangsu Provincial Innovative Research Team.
-
XS, SX, and CD conceived and designed the research.XS, JH, JG, and CW carried out the experiments.SX and CD analyzed the data.SX and CD wrote the paper.All authors have read and approved the final manuscript.
-
All the authors declare that they have no conflicts of interest.
-
All animal experiments were performed in accordance with the guidelines of the Laboratory Animal Ethics Commission of Soochow University.
Conflict of interest
Animal and Human Rights Statement
-
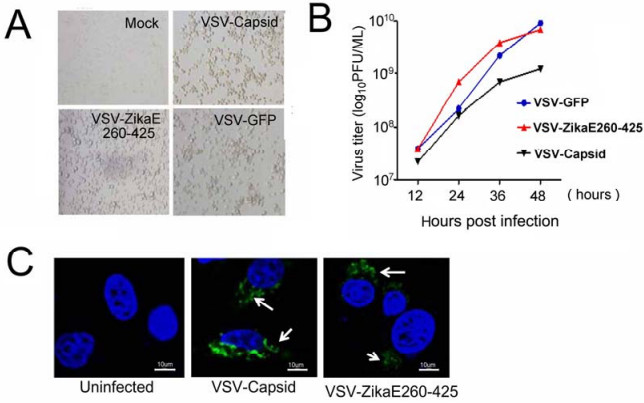
Figure S1. Construction and characterization of recombinant VSV-ZikaE260-425 and VSV-Capsid vaccines.A Microscopy images of BHK-21 cells infected with VSV-ZikaE260-425 or VSV-Capsid at 24 h postinfection.Non-infected cells were used as mock control, and VSV-GFP-infected cells were used as positive controls.B.The growth curves of VSV-GFP, VSV-ZikaE260-425 and VSV-Capsid virus.C. Immunofluorescence microscopy analysis of BHK-21 cells infected with VSV-ZikaE260-425 or VSVCapsid at 10 MOI for 12 h.Flag antibody was used to detect the ZIKV envelope and capsid proteins.Noninfected cells were used as mock controls.Scale bar=10 μm.
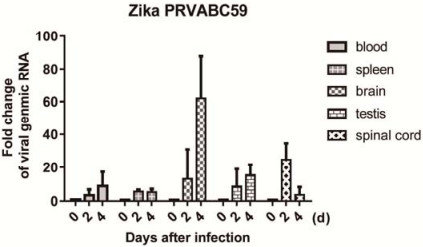
Figure S2. The relative viral genomic RNA fold change in the blood, spleen, brain, testis, and spinal cord of mice on 0, 2, and 4 days post-infection were measured by real-time PCR.Mice were intraperitoneally injected with 104 PFU ZIKV PRVABC59.The relative viral RNA level was normalized with GAPDH gene and set the level of mice on 0 day as 1.
















 DownLoad:
DownLoad: