HTML
-
Minute virus of canines (MVC) is a member of the genus Bocavirus. Bocavirus members are small, non-enveloped, autonomously replicating, single-stranded DNA (ss-DNA) viruses that belong to the subfamily Parvovirinae of the Parvoviridae family (Tattersall 2006). Up until now, besides bovine parvovirus (BPV), MVC, and human bocaviruses 1–4 (HBoV1–4) (Allander et al. 2005), several new members of bocavirus were found and reported, including gorilla bocavirus (GBoV) (Kapoor et al. 2010), porcine bocavirus (PBoV) (Li et al. 2012), feline bocavirus (FBoV) (Liu et al. 2015), sea lion bocavirus (Li et al. 2011), mink bocavirus (Yang et al. 2016), and rat bocavirus (Lau et al. 2017; Xiong et al. 2018). Usually, genome length of a parvovirus is small (approximately 5 kb). The MVC genome is composed of 5402 nucleotides, containing three open reading frames (ORF) and encoding nonstructural (NS1 and NP1) and structural proteins (VP1/VP2). Single-stranded linear DNA replication of MVC depends entirely on infection of the appropriate host cells. In our study, the Walter Reed Canine cell/3873D (WRD) cell line was permissive for MVC infection (Sun et al. 2009).
MVC was first isolated from the feces of a healthy German shepherd dog in Germany, in 1967 (Binn et al. 1970). Since then, MVC has been reported from other areas, such as Korea (Jang et al. 2003; Ohshima et al. 2004), Japan, and China (Ohshima et al. 2010; Shan et al. 2010). MVC is an important pathogen afflicting newborn pups and canine fetuses. It was reported that MVC infection caused abortion, birth deformities, and even embryonic death and resorption (Carmichael et al. 1991; Decaro et al. 2012; Carmichael 1987). The pathophysiology is thought to be associated with immune-suppression induced by MVC infection, evidenced by marked reduction in monocyte phagocytosis, viremia, interstitial pneumonia with hypertrophy and hyperplasia of alveolar cells, signs of inflammation with edema, necrosis, and infiltration of inflammatory cells in the thymus, spleen and lymph nodes (Carmichael et al. 1991; Decaro et al. 2002; Torun and Yilmaz 2005). Recently, two published reports showed that MVC infection was associated with neurological disease in dogs of various ages (Eminaga et al. 2011) and with severe gastroenteritis in an elderly dog (Ohshima et al. 2010). Based on the adverse impact on dogs and on its elusive etiology, MVC infection and the development of treatment strategies is worthy of our attention in the developed world.
Oxymatrine (OMT, C15H24N2O2) is a kind of quinolizidine alkaloid extracted from the roots of Sophoraflavescens Ait. The plant is a traditional Chinese medicinal herb, also known as kushen, used for its extensive pharmacological effects, including anti-inflammation (Guzman et al. 2013), anti-viral activity (Shi and Li 2005; Wang et al. 2011), anti-tumorigenicity (Guo et al. 2015) and antifibrosis activity (Wu et al. 2008; Yu et al. 2004). Several studies have reported that OMT shows a promising antiHBV effect in an HBV-transfected cell line, HBV transgenic mouse model, and patients with chronic hepatitis B (Lin et al. 2009). Additionally, clinical studies reported that interferon combined with OMT could significantly increase sustained anti-viral response compared with interferon alone in chronic hepatitis patients (Min et al. 2016). Recently, Dai JP et al. (2018) reported that OMT could inhibit influenza A virus (IAV) replication and IAVinduced cytopathogenic effects both in vitro and in vivo. However, a protective effect against MVC infection has not been reported. In the present study, we aimed to investigate whether OMT could protect against MVC infection in vitro.
-
OMT, a white or almost white crystalline powder with purity ≥ 98.0%, was obtained from Undersun Bio Medtech Co., Ltd. (Ningxia, China). Polyclonal antibodies against viral NS1, NP1, and VP2 were prepared in house in cooperation with Amart Company. β-actin and cleaved caspase 3 primary antibody were purchased from Proteintech Group, Inc. (Chicago, USA). Cell counting kit-8 (CCK-8) was obtained from Dojindo (Dojindo, Japan). The flow cytometry kit was purchased from Bestbio Med tech Co. (Shanghai, China).
-
The Walter Reed canine cell/3873D (WRD) cell line and MVC were gifts from Dr. Jianming Qiu, of the Medicine Department of Microbiology, University of Kansas. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco, China) supplemented with 10% fetal calf serum (FCS, BI, Israel) in 5% CO2 at 37 ℃.
-
WRD cells were cultured in 96-well culture plates (4 × 103 cells per well) and grown in DMEM at 37 ℃ in an incubator with 5% CO2. Cytotoxicity assay was performed according to the manufacturer's instructions using the Cell Counting Kit-8. Briefly, WRD cells were washed with phosphate-buffered saline (PBS) and incubated with 100 μL of OMT in a series of concentrations (0.5, 1, 2, 4, 5, 8, 10, 15 and 20 mmol/L; five wells/dilution) for 72 h. Mock-treated WRD cells served as controls. After washing with PBS, WRD cells were incubated with medium (80 μL per well) and CCK-8 solution (20 μL per well) at 37 ℃ for 1–4 h. The optical density (OD) was measured at a wavelength of 450 nm using a microplate reader (Bio-Rad, USA). The relative cell viability rate was determined for each concentration as: (OD450 drug)/(OD450 control) × 100. OMT concentrations below the 50% cytostatic concentration (CC50) were defined as non-toxic concentrations. The experiment was performed three times.
-
Based on cytotoxicity assay results, five OMT concentrations, from 0.5 to 10 mmol/L, were selected for measurement of antiviral activity using the CCK-8 assay. Briefly, WRD cells were cultured in 96-well culture plates (4 × 103 cells per well) and grown in DMEM with 10% FBS, at 37 ℃ for 16 h. After washing with PBS, cells were incubated in DMEM (without FBS) with MVC virus (MOI: 2.5, 5, 10, 20, 40, and 80, respectively) at 37 ℃, for 1 h. Medium was removed, cells were washed with PBS and incubated in DMEM (containing 10% FBS) with serial concentrations of OMT (0.5, 1, 2, 4, 5, 8, 10 mmol/L; five wells/dilution) for 72 h. The relative cell viability was determined using CCK-8 assay as described before.
-
WRD cells (1 × 106 per well) were seeded and cultured in 60 mm dishes, then infected with MVC (MOI = 10) at 37 ℃ for 1 h. Cells were washed with PBS and grown in fresh medium containing 4 mmol/L of OMT (non-toxic concentration) for 24, 36, and 48 h respectively. The cells were washed twice with PBS and lysed in 2% sodium dodecyl sulfate (SDS) followed by proteinase K (0.5 mg/mL) treatment. Hirt DNA was harvested and separated by 1% agarose gel and transferred to Hybond N+ membrane. The blots were hybridized with the pI-MVC genome probe, spanning from nt 1 to nt 5402, using the DIG High Prime DNA Labeling and Detection Starter Kit Ⅱ (Roche) according to the manufacturer's protocol. Signals were detected in the ChemiDocTM MP imaging system (BIORAD). Hirt DNA detection was carried out in Guan's lab (Wuhan Institute of Virology, CAS), and the method used was described previously (Zhang et al. 2017).
-
WRD cells (1 × 106 per well) were seeded and cultured for 16 h. Total RNA was extracted using Trizol agent and its concentration was measured using a spectrophotometer at 280 nm and 260 nm. cDNA was generated from 2 μg of RNA using a reverse transcription kit (TRAN, Beijing), and 2 μL of the diluted cDNA was subjected to RT-PCR amplification. A concentration gradient of pI-MVC plasmid was prepared by dilution up to 10 times the standard sample concentration. The primer sequences of the MVC genes are shown in Supplementary Table S1. qRT-PCR was performed using aFTC-3000P Real-Time PCR System (FunGlyn TC, Canada) with a SYBR®Green PCR Master Mix Kit (DBI®Bioscience, Germany), according to the manufacturer's instructions. mRNA expression levels were calculated using the standard curves of PCR amplification obtained from the serial dilutions of pI-MVC plasmid.
-
WRD cells (1 × 106 per well) were seeded and cultured for 16 h. The cells were collected and washed with PBS. Whole protein was extracted using a protein extraction kit (KeyGen, Jiangsu). Equal quantities of protein were subject to 10% (w/v) SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (GE Healthcare UK Ltd). Proteins of interest were identified using anti-NP1 (1:1000), antiNS1 (1:1500), anti-VP2 (1:1500) and anti-cleaved caspase 3 (1:500, Proteintech Group, Inc., Chicago, USA) primary antibodies and horseradish peroxidase-conjugated secondary antibody. Proteins were visualized by super ECL plus reagent (Applygen, Beijing, China).
-
Flow cytometry assay was used to detect cell apoptosis and analyze the cell cycle. Briefly, cells (8 × 105 cells per 60 mm dish) were seeded, infected, and cultured in DMEM with OMT (4 mmol/L) for 20 and 24 h (cell cycle analysis) or 48 h (cell apoptosis analysis). For apoptosis analysis, the cell suspension (300 μL) was incubated with 5 μL of Annexin V-fluorescein isothiocyanate (FITC) for 15 min under dark conditions at 4 ℃, then 10 μL of propidiumiodide (PI) was added in the dark and the cells were incubated for another 10–15 min at 24 ℃. Following this, the apoptotic cells were quantified using a FACSort Flow Cytometer (BD, San Jose, CA). For cell cycle analysis, the cells were collected and washed twice with ice-cold PBS. After centrifugation, the cells were fixed in 75% ethanol overnight at 4 ℃. The cells were suspended in staining solution containing 50 μg/mL PI and 0.1% ribonuclease A (RNase A) for 30 min at room temperature (24 ℃). Cell cycle was analyzed from the fluorescence profile obtained from the flow cytometer.
-
Data are shown as mean ± standard deviation (SD). All statistical analyses were made using a two-sided Student's t test, where P < 0.05 was considered significant.
Drugs and Reagents
Virus and Cell Culture
Cytotoxicity Assay
Cell Viability
Isolation of Low Molecular Weight (Hirt) DNA and Southern Blot
Quantitative Real-Time PCR (qRT-PCR)
Western Blot
Flow Cytometry
Statistical Analysis
-
Cytotoxicity assay was performed using a CCK-8 kit. The results showed that relative cell viability was above 95% upon treatment with OMT at concentrations of 0.5, 1, 2, 4, and 5 mmol/L, and at these concentrations, the drug had no effect on cell morphology when compared with mocktreated cells (Fig. 1). The 50% cytostatic concentration (CC50) was 10 mmol/L. Therefore, the maximal safety concentration for OMT was ascertained as 4 mmol/L, which was the nontoxic concentration used for subsequent antiviral assays.
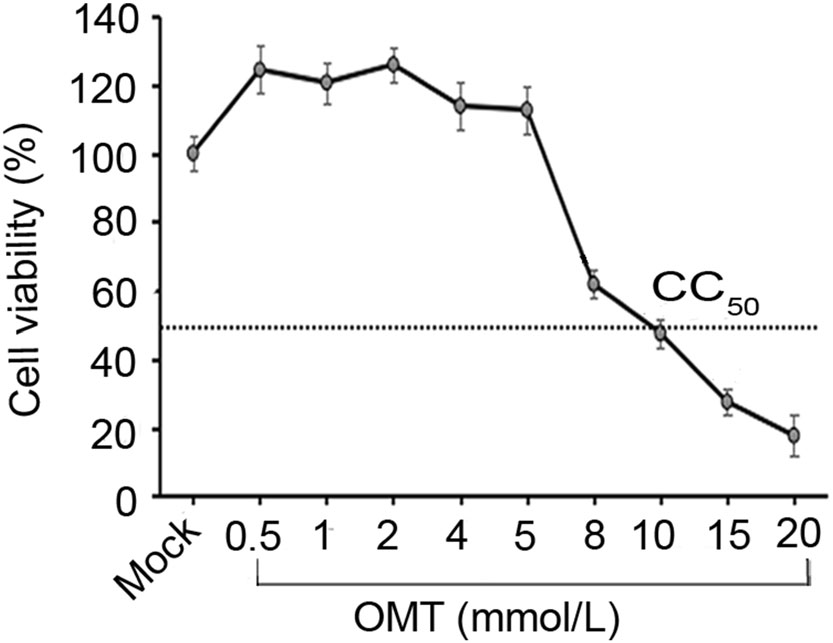
Figure 1. Determination of the nontoxic concentration of OMT in WRD cells. Cells were treated with a series of concentrations (0.5, 1, 2, 4, 5, 8, 10, 15, 20 mmol/L) of OMT for 72 h. CCK-8 assay was used to detect optical density (OD) at 450 nm. The relative cell viability was calculated as: (mean OD450 OMT)/(mean OD450 control) × 100%. Data were shown as mean ± SD of three independent experiments. CC50 value of OMT is shown by a dotted line.
-
To observe the effect of OMT on MVC-infected cell viability, 0.5–10 mmol/L OMT was administrated in the MVC-infected group (MOI: 10). As shown in Fig. 2A, OMT within the nontoxic concentration range could increase the viability of MVC-infected cells (P < 0.05) with a slightly dose-dependent effect when compared with the MVC-infected group. To further observe the effect of OMT on MVC infection with different MOI, the maximal safety concentration of OMT (4 mmol/L) was used. The result indicates that cell viability in MVC-infected group significantly decreased, compared with that of the mock group (P < 0.05). However, cell viability in the MVC/OMT group was significantly higher than that in the MVCinfected groups when using an MOI of 5 and 10 (P < 0.01) (Fig. 2B). With increased MVC infection, however, the ability of OMT to protect cell viability declined. These results indicate that OMT enhanced the viability of MVCinfected cells within a range of MOI.
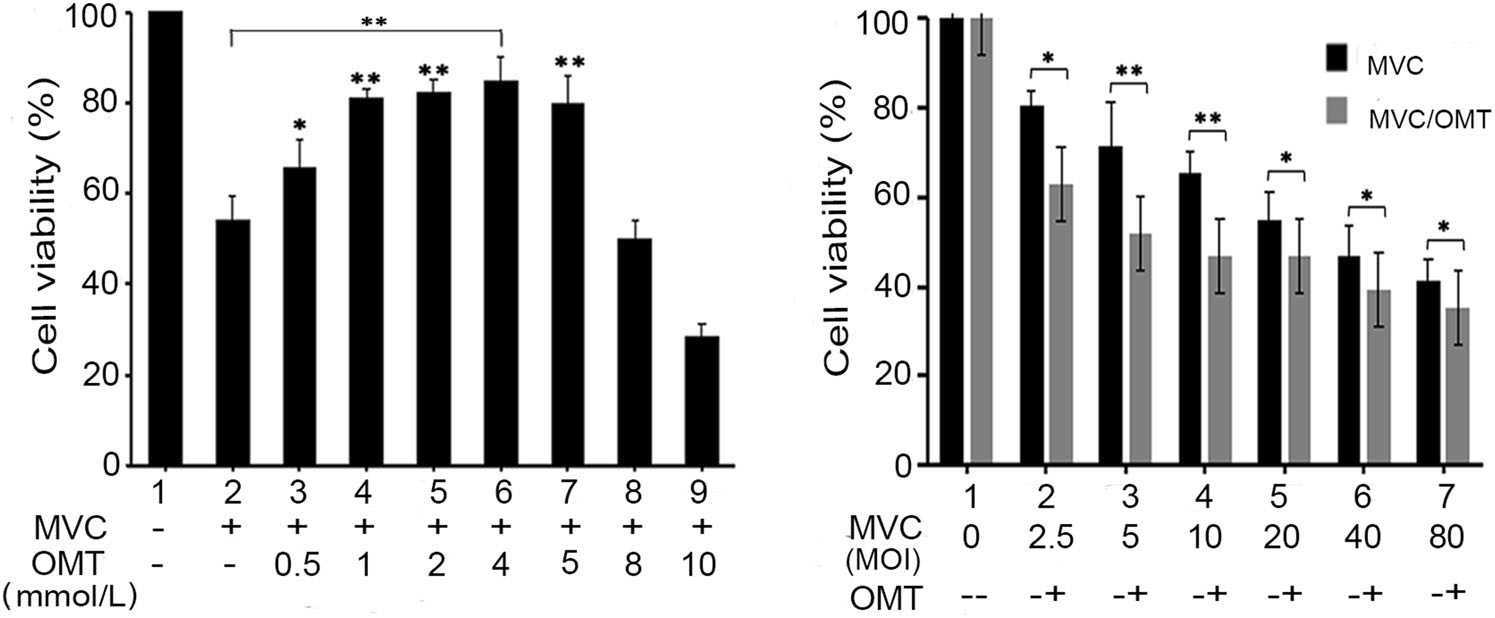
Figure 2. OMT enhances the viability of MVC-infected cells. A Cells were infected with MVC (MOI:10) and treated with different concentrations (0.5, 1, 2, 4, 5, 8, 10 mmol/L) of OMT. B Cells were infected with MVC at different MOI values (2.5, 5, 10, 20, 40, 80) and treated with OMT (4 mmol/L, the maximal safety concentration). After 72 h, optical density (OD) was detected by CCK-8 assay. The data are shown as mean ± SD of three independent experiments. *P < 0.05; **P < 0.01.
-
Given that OMT enhanced the viability of MVC-infected cells, we speculated whether this function was associated with MVC replication. First, to evaluate the effect of OMT on MVC DNA replication, the viral genome of MVC in infected WRD cells was extracted and analyzed by Southern blot assay. As shown in Fig. 3, there was no replication band in either MVC-infected or OMT-treated groups 12 h post-infection, suggesting that the virus is in its infective entering phase or that few viruses are entering the DNA replication phase. However, 24 and 48 h postinfection in MVC-infected cells, viral DNA replication was detected. We found that in the OMT-treated group, MVC DNA replication form (RF) was decreased at 24 h (P < 0.01) and 48 h (P < 0.05) post-infection, when compared with the MVC-infected group. RF DNA was reduced by approximately two- to three-fold relative to the MVC-infected group (Fig. 3). On the other hand, ssDNA (single-stranded DNA) levels of the MVC genome were no difference between the MVC-infected and OMT-treated groups. Viral ssDNA in infected cells is usually seen upon viral entry and uncoating within the host cells. Clearly, our results confirmed that OMT enhances the viability of MVC-infected cells by inhibiting MVC DNA replication.
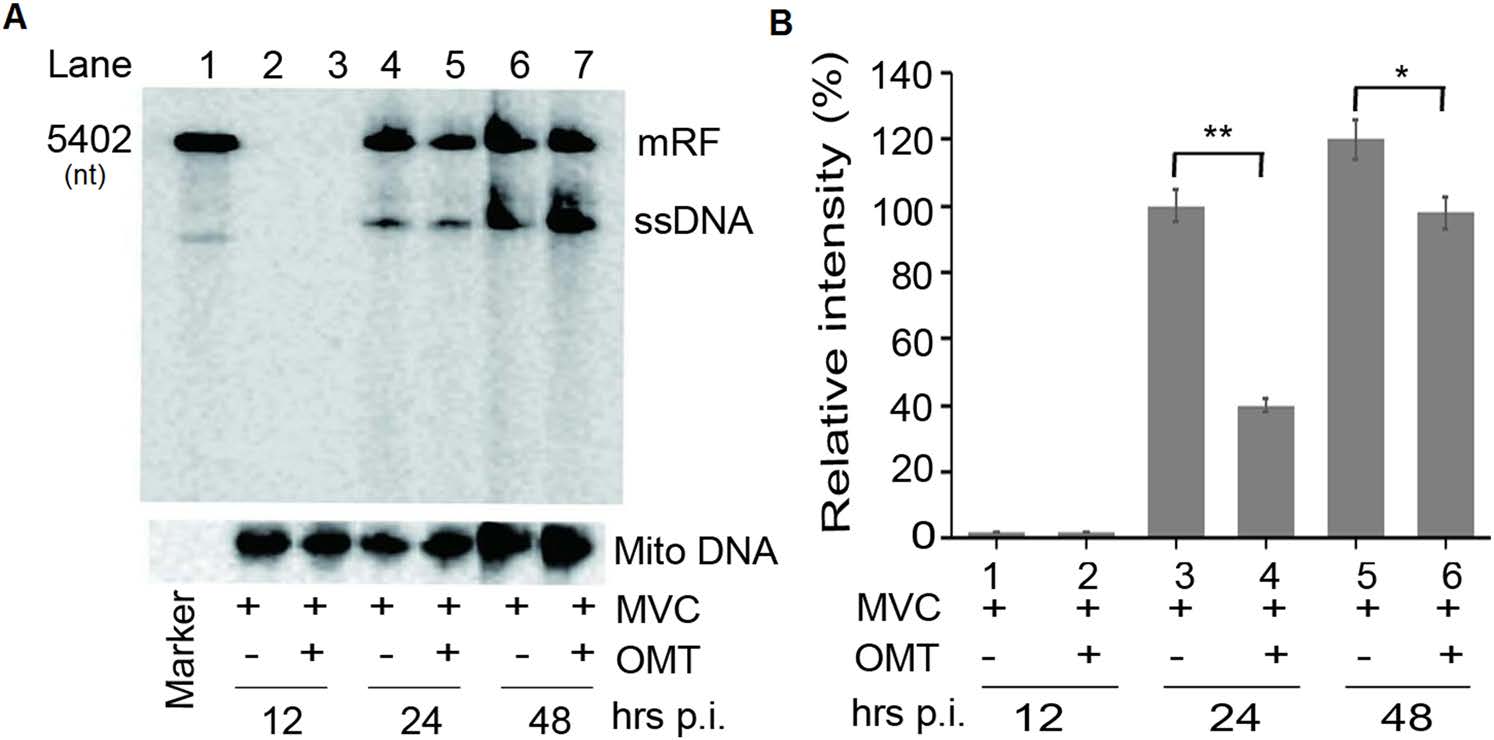
Figure 3. OMT reduces MVC viral DNA replication. WRD cells were infected with MVC (MOI: 10) at 37 ℃ for 1 h. Medium was replaced with fresh medium containing OMT (4 mmol/L) for 12, 24, 36, and 48 h. Hirt DNA was harvested and detected by Southern blot. A Southern blot assay was performed to analyze viral DNA replication. B Statistical analysis of replication bands using Image J was performed, with the relative intensity value expressed as percentage of Mito-DNA. mRF, mono MVC DNA replication form. The data are shown as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
Parvovirus nonstructural proteins (NS1 and NP1) and structural proteins (VP1/2) are essential for viral DNA replication, virion generation, and infection (Sun et al. 2009). Therefore, the next logical step was to confirm the inhibitory effect of OMT by measuring the expression levels of NS1, NP1, and VP1/2 genes. mRNA and protein levels were determined using qRT-PCR and western blot assays respectively. For qRT-PCR, the total viral RNA synthesized in infected cells was determined at time-points 24 h, 36 h, and 48 h post-infection. As shown in Fig. 4A, the administration of OMT led to a significant reduction in the expression of viral NS1, NP1, and VP1/2 at an mRNA level (**P < 0.01 or *P < 0.05), especially at 36 h and 48 h post-infection. NS1 and NP1 mRNA expression levels were approximately five-fold and two-fold lower in the OMT treated group than in the MVC infection group at 36 h and 48 h post-infection respectively.VP1/2 mRNA expression was also inhibited about two-fold in the OMT treated group relative to the MVC-infected group at 36 h and 48 h post-infection, respectively (Fig. 4A). Viral NS1, NP1, and VP1/2 proteins were expressed significantly 24 h following MVC infection; however, their levels were reduced (P < 0.05) in the OMT-treated group (Fig. 4B). These results indicate that OMT inhibits DNA replication of MVC, which is associated with the decreased expression of crucial viral genes.

Figure 4. OMT reduces viral gene expression in MVC-infected cells. WRD cells were seeded, infected, and treated for detecting viral gene expression. A qRT-PCR assay was used to analyze the mRNA expression levels of viral NP1, NS1, and VP2 genes. A concentration gradient of pI-MVC plasmid was prepared by 10 times dilution as the standard sample. The expression levels were calculated using pIMVC plasmid copy number. B The changes of protein expression for viral NP1, NS1, and VP2 were investigated by Western blot (a). Statistical analysis of bands showing protein expression levels was performed using Image J (b). Relative intensities were represented as a percentage of β-actin band intensities. The data are shown as mean ± SD for three independent experiments. *P < 0.05, **P < 0.01.
-
It is generally accepted that viral DNA amplification of autonomous parvoviruses relies on the entry of host cells into S phase of their cell cycle (Luo et al. 2013a, b). MVC infection induces accumulation of infected cells in the S phase during early infection, which supports active viral DNA replication (Luo et al. 2011, 2013a, b). Having demonstrated that OMT inhibits DNA replication of MVC and decreases viral gene expression, we next sought to analyze whether this effect was mediated through host cell cycle progression. Changes in cell cycle progression were examined in MVC-infected cells and in infected cells treated with OMT (OMT-treated group) 20 h and 24 h post-infection. As shown in Fig. 5, approximately 11% and 8.71% of mock-infected cells were in S phase at 20 h and 24 h post-infection, respectively. On the other hand, 43.42% and 30.34% of MVC-infected cells were in S phase 20 h and 24 h post-infection respectively, indicating that MVC infection induces accumulation of infected cells in S phase during early infection, which was consistent with previous reports (Luo et al. 2013a, b). In comparison with MVC-infected cells, the distribution of OMT-treated cells in S phase decreased from 43.42% to 35.08% 20 h postinfection, and from 30.34% to 22.83% 24 h post-infection (Fig. 5). Collectively, these results confirmed that OMT reverses S-phase arrest induced by MVC during early-stage infection.
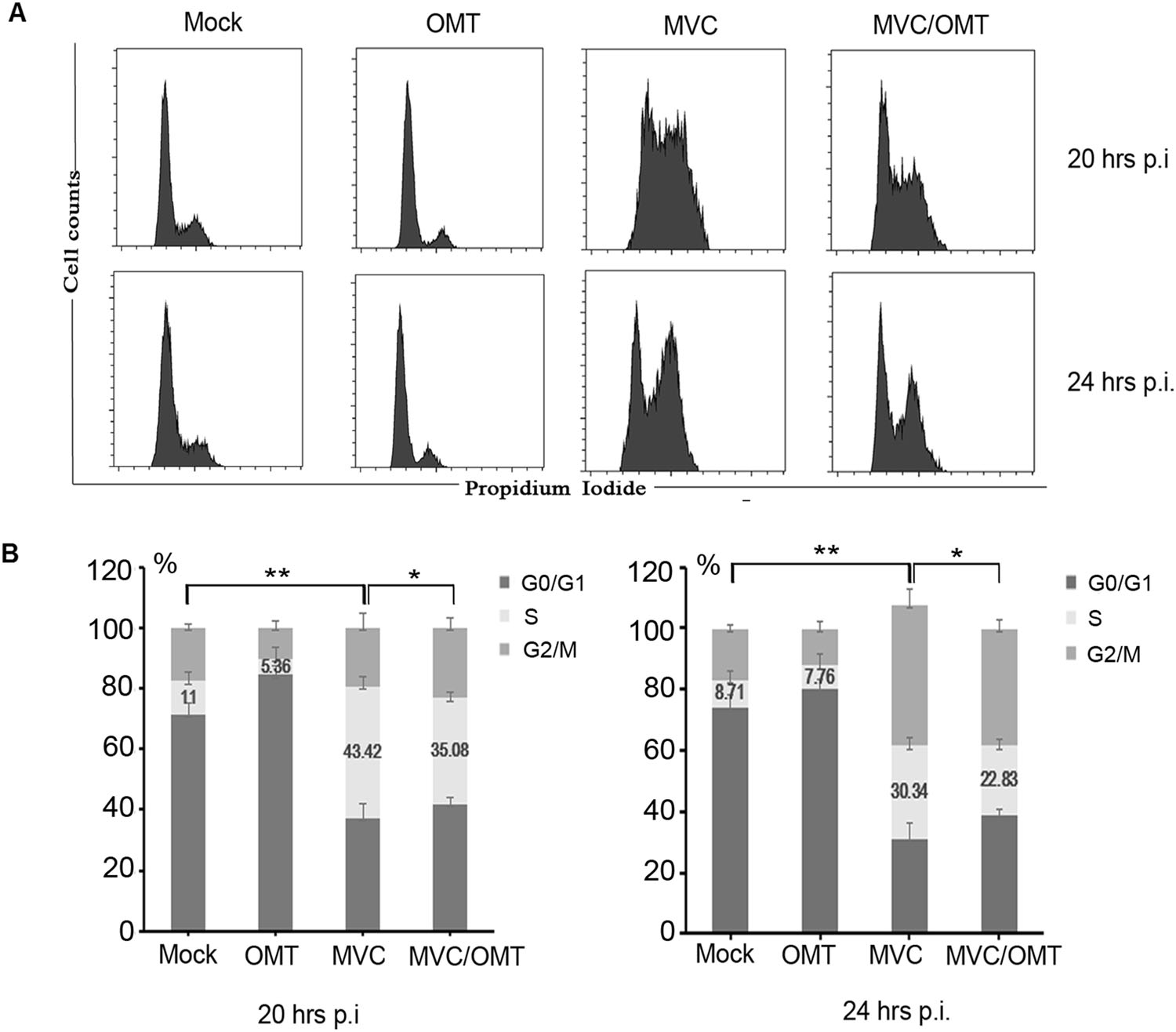
Figure 5. OMT decreases S-phase arrest induced by MVC infection at early stages of infection. WRD cells were seeded, infected, and treated with OMT for 20 and 24 h. Cells were fixed with 75% ethanol and stained with PI, then detected by flow cytometry (A). Statistical analysis was done using ModiFit LT software (B). The percentage of cells at each cell cycle phase was quantified in a column chart as indicated. *P < 0.05, **P < 0.01.
-
MVC induces cell cycle arrest in S phase during early infection, in G2/M phase at later stages, and finally induces mitochondrion-mediated apoptosis in host cells (Chen et al. 2010; Luo et al. 2011). As shown in Fig. 5, OMT reduced S-phase arrest in early stages of MVC infection. Next, we sought to observe whether OMT is also able to abrogate MVC-induced host cell apoptosis. Following infection, cells were incubated with OMT for 48 h, and cell apoptosis was detected by flow cytometry. The results showed that the proportion of apoptosis in MVC-infected cells increased from 1.8% (WRD, mock-infected cells) to 42.5% (Fig. 6), which is consistent with previously published findings (Chen et al. 2010). Interestingly, OMT treatment reduced host cell apoptosis from 42.5% in MVC-infected cells to 26.7%. Taken together, these results show that MVC-induced host cell apoptosis in later stages of infection was significantly reduced by OMT treatment.

Figure 6. OMT reduces host cell apoptosis induced by MVC infection. WRD cells were seeded, infected, and treated with OMT for cell cycle analysis. A Cells were stained with FITC and PI 48 h post infection. Flow cytometry was performed to examine the percentage of live cells, dead cells, and apoptotic cells. Data were listed in the table as indicated. B Statistical analysis of cell apoptosis rate in four groups. Data are shown as mean ± SD of three independent experiments. **P < 0.01.
Apoptosis is the end result of an elaborate cascade of molecular events that ultimately result programmed cell death. Based on the flow cytometry assay results, we decided to check for levels of activated cleavage product of caspase 3 using Western blot. As shown in Fig. 7, the activated cleavage product of caspase 3 was present in MVC-infected cells at 36 h and 48 h post-infection. By contrast, cleaved caspase 3 was significantly reduced in OMT-treated cells relative to that in MVC-infected cells. Our results indicate that OMT has anti-MVC activity associated with inhibition of DNA replication, decreased viral gene expression, reduced S-phase arrest at early stages of infection, and reduced apoptosis at later stages.
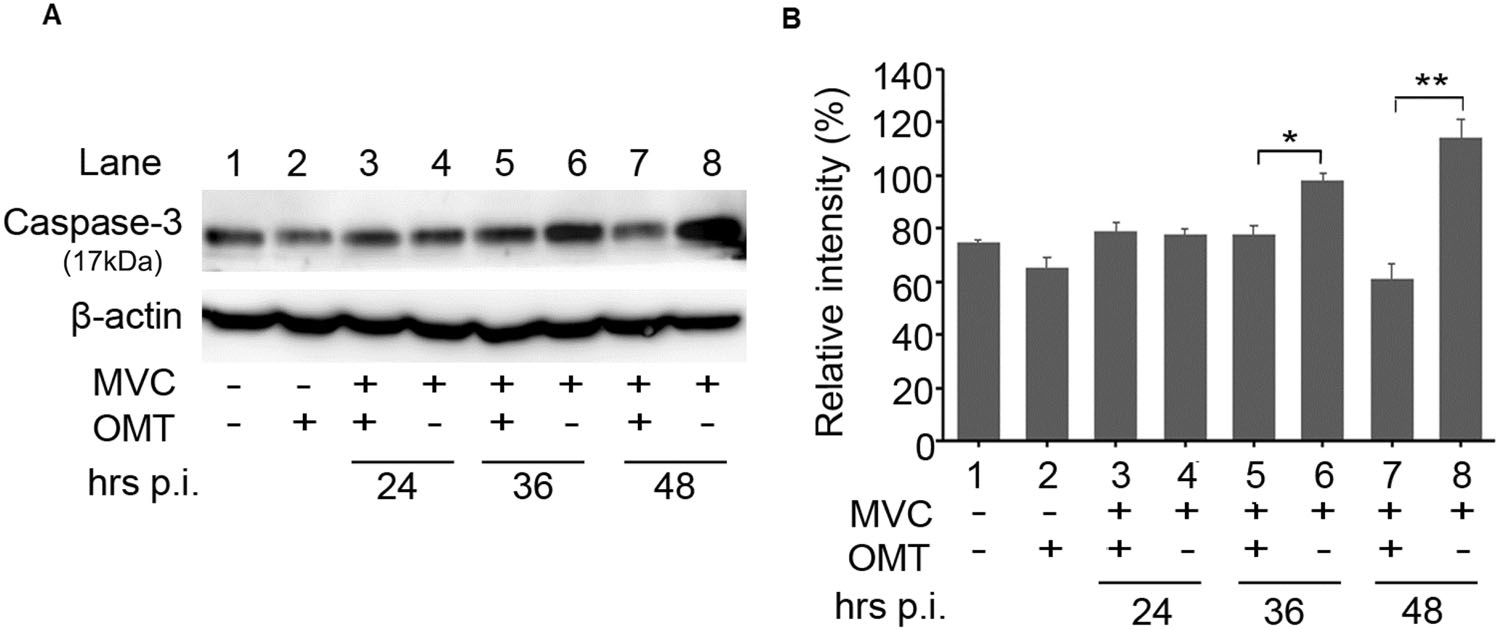
Figure 7. OMT reduces expression of cleaved caspase 3 in MVCinfected cells. WRD cells were seeded, infected, and treated for performing Western blot. A The expression of cleaved caspase 3 (17 kDa) and β-actin were detected by western blot. B Statistical analysis of the expression of protein bands was performed using Image J. Relative band intensities were represented as percentages of β-actin band intensities. Data were shown as mean ± SD of three independent experiments.*P < 0.05; **P < 0.01.
OMT Cytotoxicity Assay
Effect of OMT on MVC-Infected Cell Viability
Effect of OMT on MVC Replication and Gene Expression
Effect of OMT on Cell Cycle
Effect of OMT on Cells Apoptosis
-
Traditional Chinese medicinal herbs (TCMHs) have long been used for the prevention and treatment of viral infectious diseases in China and many other Asian countries. So far, a growing number of TCMHs are also garnering interest as potential antiviral agents (Li and Peng 2013). Several antiviral TCMHs have been approved by the China Food and Drug Administration (SFDA), including Radix bupleuri, Fructusforsythiae, Radix isatidis, and Sophoraflavescens (Li and Peng 2013). OMT is one of the major alkaloid components found in Sophoraflavescens Ait. Several reports (Jiang et al. 2017) have demonstrated that OMT and similar compounds have anti-tumorigenic, antiatherosclerotic, anti-viral, anti-parasitic, immunosuppressive, hepatoprotective, cardioprotective, and neuroprotective activity.
In this study, for the first time, we have reported that OMT is a suppressor of MVC DNA replication and viral gene expression via reversal of MVC-induced host cell accumulation in the S-phase of the cell cycle during early stage infection. Moreover, OMT also increased cell viability, decreased MVC-infected cell apoptosis and reduced pro-apoptotic cleaved caspase 3 expression. This study on the anti-MVC activity of OMT could help us to elucidate the effect of OMT on infection caused by other parvoviruses.
Like other parvoviruses, MVC requires actively growing cells for viral DNA replication. WRD cells are permissive for MVC infection, so MVC DNA could replicate and form infectious progeny in them. It is generally accepted (Wolter et al. 1980) that actively dividing cells acquire some mechanism necessary for viral replication which is absent from quiescent cells, and which cannot be induced by this very simple virus genome. In addition, it also appears likely that (Oleksiewicz and Alexandersen 1997; Luo et al. 2013a, b) parvovirus DNA replication relies on host cell S phase for viral DNA amplification. Consistent with previous reports (Chen et al. 2010; Luo et al. 2013a, b), our results showed that MVC infection induces accumulation of infected cells in the S phase 20 h post-infection (Fig. 5). Viral replication represents the core of the viral life cycle, and involves most viral protein functions and cellular machinery. Thus, development of TCMHs with antiviral activity is focused principally on the viral replication stage of infection (Li and Peng 2013). Wang et al. (2011) reported that OMT inhibited HBV DNA replication and HBeAg production by down-regulating the expression of heat-stress cognate 70 (Hsc70), and Liu et al. (2018) also showed that OMT had potent inhibitory effects on both wild-type and entecavir-resistant HBV in vitro and effectively suppressed HBV replication in a mouse model. Moreover, Dai showed that OMT could inhibit IAV replication and inflammation via regulation of toll-like receptor 4 (TLR4), p38 mitogen activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) pathways (Dai JP et al. 2018). In the present study, the results showed that OMT could decrease MVC DNA replication. It is generally accepted that parvovirus NS1 is a multifunctional polypeptide that is essential for the replication of the viral genome. Our previous study confirmed that NS1 and NP1 are essential for MVC genome replication in WRD cells (Sun et al. 2009), based on our finding that the replication of MVC DNA of the NS1(-) mutant was totally abolished. Moreover, without NP1, replication of MVC DNA was significantly reduced by 320-fold. In this study, we found that OMT decreased the expression levels of MVC NS1 and NP1 (Fig. 4), suggesting that OMT was able to reduce MVC DNA replication. Whether the antiMVC activity of OMT is involved in other signaling pathways in host cells is unclear and needs further study.
Parvovirus infection often causes death of infected cells through apoptosis or non-apoptotic cell death. Apoptosis is mechanistically categorized into two major pathways: the mitochondrion-mediated (intrinsic) pathway and "death" receptor-mediated (extrinsic) pathway. Both pathways involve the sequential activation of caspases. Many studies have reported (Chen and Qiu 2010; Doley et al. 2014; Zhang et al. 2018) that parvovirus infection usually induces apoptosis, including infection by porcine parvovirus, human parvovirus B19, canine parvovirus, parvovirus H-1, and MVC. Our previous study showed that MVC infection induced mitochondrion-mediated apoptosis, represented by the presence of activated caspases in infected cells (Chen et al. 2010). Consistent with this, our results from this study also confirmed that MVC infection induced apoptosis at later stages, and that caspase 3, the effector caspase, was activated during MVC infection (Fig. 7). However, OMT was shown to decrease host cell apoptosis induced by MVC infection and reduce the expression of activated caspase 3. Many published reports (Liu et al. 2014; Dai Z et al. 2018) have shown that OMT has antitumor activity in various cancer cell lines mediated by induction of cell cycle arrest and apoptosis. However, there are few reports on antiviral activity of OMT associated with cellular apoptosis. In the present study, for the first time, we have demonstrated OMT activity against MVC parvovirus that is associated with regulation of host cell apoptosis.
In summary, OMT reduced MVC DNA replication through inhibition of cell cycle S-phase arrest in the early stages of MVC infection. OMT also decreased MVC-infected cell apoptosis and reduced the expression of proapoptotic cleaved caspase 3. Our results suggest that OMT has potential application in the clinical treatment of parvovirus infection.
-
We are thankful to Professor Jianming Qiu (Department of Microbiology, Molecular Genetics and Immunology, University of Kansas Medical Center, USA) for providing WRD cells and bocavirus MVC, and Huanzhou Xu (a member of Guan's lab, Wuhan Institute of Virology, CAS, China) for technical help, and Xiangli Hao (School of Foreign Languages, Ningxia Medical University, China) for his assistance in language polishing. This work was funded by the Natural Sciences Foundation of China (31760041) to YS, the West China first-class Disciplines Basic Medical Sciences at Ningxia Medical University (No. NXYLXK2017B07) and Innovative Training Program for College Students (201510752010) to NL.
-
YS conceived/designed the experiments. YD, NL and JS performed the experiments and analyzed the data. JS, LZ, JG and XH contributed reagents/materials/analysis tools. YS and YD wrote the manuscript. YD and NL prepared the figures and tables. YS checked and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Author Contributions
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
Animal and Human Rights Statement
-

Table S1. Primer Sequences and Sites in the MVC genome
















 DownLoad:
DownLoad: