-
Dear Editor,
Hepatitis C Virus (HCV) infection is a huge public health problem globally, because it can lead to adverse long-term clinical outcomes. In China, the population has experienced a skyrocketing growth of HCV infections because of paid blood donations in the late 1980s to early 1990s (Lu et al. 2013; Yin et al. 2015). Fortunately, the prevalence of HCV infection has declined dramatically since mid-1990s and was less than 1% in recent years (Cui and Jia 2013; Fu et al. 2010). However, the huge size of population in China makes a quite enormous absolute number of people infected with HCV. Like other agents causing blood-borne diseases, HCV is mainly transmitted through exchange of bodily fluids and intravenous drug use, as well as vertical transmission (Lauer and Walker 2001). Compared to the more in-depth understanding on infection and virus transmission in high-risk groups, limited data are available concerning the HCV infection caused by infrequent exposure in general population. Herein, we documented two sisters with chronic HCV infection and our attempts to dissect the transmission routes of their infections.
The two patients went to Department of Infectious Disease of the Fourth Hospital of Harbin Medical University in Heilongjiang Province of China seeking for medical advice for the reason that they felt weakness and fatigue without obvious inducement. The elder sister was 48 years old, she stated that she was detected HCV antibody-positive in routine annual physical exams 3 years ago. The younger one was 45 years old and was diagnosed with HCV infection half a year ago. Results of quantitative PCR showed that the elder sister's HCV-RNA load was 6.27 × 105 IU/mL and the younger sister's was 1.11 × 106 IU/mL. HCV genotyping revealed that both were infected with the genotype 2a virus. None of them had received any anti-HCV therapy before. Biochemical examination revealed that the elder sister displayed a slight increase in alkaline phosphatase (ALP), total bile acids (TBA) and hyaluronan (HA) (Table 1). The younger sister displayed a slight elevation of total bilirubin (TBIL) and indirect bilirubin (IBIL) levels (Table 1). Additionally, the younger sister seemed to show metabolic disorders in glucose and lipids. In sum, the signs of liver damage were seen in both sisters, suggesting a result of chronic HCV infection. Tests of antibody against HBV and autoimmune antibodies (including anti-nuclear antibody, anti-centromere antibody, smooth muscle antibody, anti-liver-kidney microsomal antibody, anti-mitochondrial antibody, anti-mitochondrial antibody M2) were all negative, excluding the possibility of autoimmune hepatitis or primary biliary cirrhosis.
Laboratory test Normal value (NV) Elder Younger Nov. 26, 2018 Dec. 04, 2018 Nov. 26, 2018 Dec. 04, 2018 Blood routinetest WBC 3.5–9.6×109/L 4.13 3.63 3.20 3.93 NEUT 1.8–6.3×109/L 2.21 1.80 2.96 1.91 LYMPH 1.1–3.2×109/L 1.50 1.41 1.87 1.74 Mono 0.1–0.6×109/L 0.31 0.37 0.27 0.25 Eos 0.02–0.52×109/L 0.08 0.05 0.10 0.03 Bas 0–0.06×109/L 0.03 0 0 0 RBC 3.8–5.1×1012/L 4.92 4.89 4.69 4.72 HGB 115–150 g/L 137 136 132 135 PLT 125–350×109/L 169 141 181 164 CHO 2.4–5.17mmol/L 4.01 - 4.09 - TG 0.4–2.3 mmol/L 1.95 - 3.93 ↑ - HDLC 1.2–1.68mmol/L 1.03 - 0.87 ↓ - LDLC 2.07–3.1mmol/L 2.07 ↓ - 1.91 ↓ - GLU 3.89–6.11mmol/L 4.61 - 6.42 ↑ - Biochemicalparameters ALT 7–40 U/L 17 15 24 27 AST 13–45 U/L 20 19 21 18 TBIL 3.4–17.1μmol/L 12.4 6.6 19.8 ↑ 8.5 DBIL 0–6.8 μmol/L 3.6 2.1 4.9 3.2 IBIL 1.7–13.5μmol/L 4.5 4.5 14.9 ↑ 5.3 GGT 7–45 U/L 17 16 28 23 ALP 35–100 U/L 134 ↑ 106 ↑ 71 59 ALB 40–55 g/L 43.0 40.6 47.9 44.2 GLO 20–40 g/L 27.8 23.2 27.2 25.0 TBA 0–10 μmol/L 12.7 ↑ 7.70 3.20 5.90 BUN 2.8–8.2mmol/L 3.98 - 3.69 - CREA 42–104μmol/L 65.2 - 57.9 - HA 0–100 ng/L 196.3 ↑ - 65.0 - LN 0–50 ng/L 25.2 - 25.1 - PIIIP 0–30 ng/L 17.1 - 17.4 - C-IV 0–30 ng/L 11.3 - 12.3 - WBC, white blood cell; NEUT, neutrophil; LYMPH, lymphocyte; Mono, monocyte; Eos, eosinophil; Bas, Basophil; HGB, hemoglobin; PLT, platelet; BUN, blood urea nitrogen; CREA, creatinine; TC, total cholesterol; TG, triglyceride; HDLC, High density lipoprotein cholesterol; LDLC, Low density lipoprotein cholesterol; GLU, glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; IBIL, indirect bilirubin; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; ALB, albumin; GLO, globulin; TBA, total bile acids; HA, Hyaluronan; LN, laminin; PIIIP, Procollagen III N-peptide; C-IV, type IV collagen.
↑: Value higher than the normal range; ↓: Value lower than the normal range.Table 1. Laboratory characteristics of the patients.
Partial HCV sequences were recovered by nested RTPCR within the E1 and NS5B genes (corresponding to nucleotides 739 to 1310 and 8267 to 8630, respectively, in the H77 genome). The partial E1 and NS5B sequence identities between the two sisters were 86.8% and 92.3% for nucleotide and 91.6% and 95.9% for amino acid respectively. These data suggested that they were not from the same origin. Sequences recovered in this study and reference sequences were used to perform phylogenetic analysis using the maximum likelihood method implemented in PhyML version 3.0 (Guindon et al. 2010). To reduce the sampling biases, we chose the HCV genotype 2a strain from previous study on migration patterns of hepatitis C virus in China (Lu et al. 2014). Also, top 2 BLAST and HHblits hits were included. Sequences from outside China were obtained from the Los Alamos HCV database (http://hcv.lanl.gov). All HCV sequences from China were divided into five groups (north-northeast, northwest, central south, southeast and southwest), according to the geographical origin (Lu et al. 2014; Peng et al. 2015). The phylogenetic tree of NS5B (Fig. 1A, 1B and Supplementary Figure S1 for comprehensive image analysis) showed that the younger sister's virus grouped with the strain KC967476 from Denmark, then clustered with the elder sister's virus. Both their partial NS5 did not clustered the top BLAST hits (GZ215 for the elder sister's and BJ665 for the younger's) (Fig. 1B). As shown in the phylogenetic tree of E1, the elder sister's sequence was grouped with BJ15 from north-northeast of China (Fig. 1C, 1D and Supplementary Figure S2 for comprehensive image analysis), and then joined the group in which the strain ZS665 (the top BLAST hits for the elder's) localized. Whereas the HCV strain from the younger sister clustered with the strain SH48 from the southeast of China but fell into different group with the top BLAST hits AF169004 from Japan. Notably, the viral sequences from the two sisters didn't show geographic clustering with the sequences from adjacent provinces, reflecting the complexity of the dissemination pattern of HCV subtype 2a.
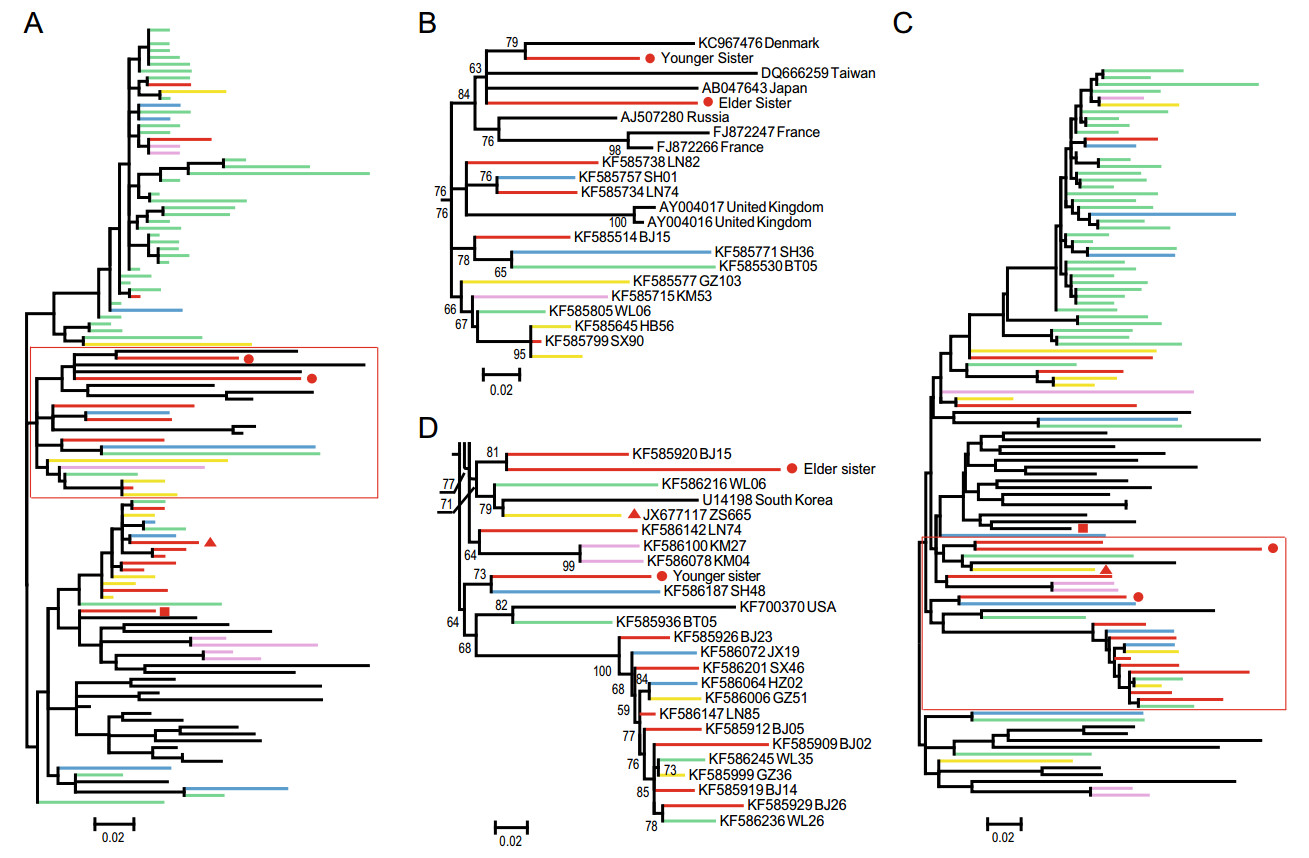
Figure 1. Phylogenetic relationships of the partial NS5 and E1 gene of HCV. A. Phylogenetic tree based on partial NS5 gene. B. Detail of the sequence relationships in the red rectangle area in A. C. Phylogenetic tree based on partial E1 gene. D. Detail of the sequence relationships in the red rectangle area in C. The tree is mid-point rooted for clarity. Bootstrap values > 50% are shown at relevant nodes. Branches are colored according to their geographic origins, indicated at the bottom. The meaning represented by symbol '●, ▲ and ■' were also indicates at the bottom. Sequences reported in this study have been submitted to GenBank and assigned the accession numbers MH463463–MH463466.
To better understand their disease histories, epidemiologic investigation was performed. Members of the immediate family of the two sisters including their parents, husbands and children were screened for HCV by quantitative PCR and serologic tests (IgM and IgG), none of them was HCV-positive. Finally, the younger sister had surgery for subarachnoid hemorrhage in 2015, yet both sisters denied a history of blood transfusion, intravenous drug use and experience of unprotected sex other than with a spouse. Unfortunately, the attempts to dissect the transmission route were failed. The only significant risk factor of the younger sister was the surgery. However, infrequent exposure could not be ruled out. As for the elder sister, it most likely not to be infected through the high-risk factors.
Both sisters received antiviral treatment starting the day after admission (day 2). They were treated with peginterferon alpha-2a 180 μg subcutaneous injection once a week plus ribavirin 900 mg oral daily for 24 weeks. On day 7, another routine blood and liver function test were performed. The ALP and TBA of the elder sister dropped slightly toward the normal range; the younger's ALP also dropped slightly and the TBA increased a little though still within the normal range. The high TBIL and IBIL of the younger sister had returned to normal (Table 1). The results suggested that their liver function had gradually improved. Both sisters were discharged from the hospital on day 12. The anti-viral medication continued, and they were monitored on an outpatient basis after discharge. At the end of treatment, HCV viral RNA was not detected by real-time PCR test from their blood samples. Also, they were arranged follow-up every 6 months for 3 years, HCV viral RNA was not detected by real-time PCR test every time.
Although hepatitis, jaundice and even fulminant hepatic failure can occur in the early stage of HCV infection (Hoofnagle 1997; Cox et al. 2005; Villano et al. 1999; Mosley et al. 2005), overall, one-third of patients with acute infection are symptomatic (Hoofnagle 1997; Cox et al. 2005). In our study, two sisters claimed no discomfort until 1 week before hospitalized they felt fatigue. Hence, like most HCV-infected persons, they were unaware of their status, although they are at risk for life-threatening diseases such as cirrhosis and hepatocellular carcinoma (HCC) (Hoofnagle 1997).
The two sisters have never donated their blood so most likely they were not the direct victims of the illegal blood donation campaign. Also they claimed not to be intravenous drug users and have no experience of unprotected sex other than with a spouse. They also denied any other nonmedical percutaneous exposures such as tattooing and piercing. The younger sister has had surgery for subarachnoid hemorrhage. However, she did not receive any blood transfusion during the operation, which is the only significant risk factor of the younger sister infected with HCV. Since the operation was done 3 years ago, it is unable to trace. As for the elder sister, there was no identifiable risk factor for her HCV infection. It could be unsafe medical procedures, nonmedical percutaneous exposures or other infrequent transmission mode, but most likely we shall never learn about the conclusive evidence.
In the developed countries, the rapid improvement of healthcare conditions and the introduction of anti-HCV screening for blood donors have led to a sharp decrease in the incidence of iatrogenic hepatitis C, but the epidemic continues to spread in developing countries. Although the prevalence of HCV has declined in China (Cui and Jia 2013), the total HCV-infected population is still considerable. Since most of the chronic HCV infection will progress to end-stage liver diseases (Hoofnagle 1997), the economic burden of disease caused by the virus is enormous. The prevention and control of HCV remains a major public health problem in China. It is well known that HCV is transmitted primarily through infectious blood or body fluids that contain blood. Additionally, HCV could be transmitted frequently through intravenous drug use through the sharing syringes/needles and reusing inadequately sterilized medical equipment, organ transplant or transfusions of blood and blood products, and unsafe sexual activity. However, HCV infection caused by infrequent transmission mode (e.g. beauty treatments, piercing, acupuncture, sharing personal items, cohabitant HCV-positive) was also reported in China (Li et al. 2017) and other country (Karmochkine et al. 2006; Kiyosawa et al. 1994; Mariano et al. 2004). Our data also suggest that both sisters might be infected through the infrequent exposure. In sum, HCV infection caused by infrequent exposure could contribute to HCV spread and therefore should be paid more attention to it.
-
This study was supported by the National Natural Science Foundation of China (Grants 81861138003 and 81672057).
-
LYZ and YZZ designed the study. XCQ and LHZ performed the experiments. LHZ and LYZ collected clinical data in this study. XCQ, AP and YZZ analyzed the data. XCQ, AP and YZZ drafted the manuscript. All authors interpreted the results and approved the final manuscript.
HTML
Acknowledgements
Author Contributions
-
The authors declare that they have no conflict of interest.
-
The investigation was reviewed and approved by the Ethics Committees of the Fourth Hospital of Harbin Medical University and the Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Additional informed consent was obtained from all patients for which identifying information is included in this article.
















 DownLoad:
DownLoad: