HTML
-
Human adenovirus (HAdV) is a wide spectrum virus that causes multiple systemic infections, such as acute respiratory disease (ARD), epidemic keratoconjunctivitis, hemorrhagic cystitis, gastroenteritis, as well as other diseases (Lion 2014; Zhao et al. 2014). Using the whole genome sequence as a means of typing, 103 genotypes are currently recognized and classified into seven species A–G (Seto et al. 2011; Zhang et al. 2017). Among the genotypes of HAdV causing ARD, type 3 (HAdV-3) is the most common one reported to WHO (Echavarría 2009); it is particularly prevalent among children. Outbreaks of HAdV-3 infections are reported globally and recently, including the United States (Lebeck et al. 2009), Brazil (Pereira et al. 2016), the United Kingdom (Alkhalaf et al. 2015), Korea (Lee et al. 2010), Australia (Harley et al. 2001), Canada (Yeung et al. 2009), Singapore (Coleman et al. 2019), Malaysia (Li et al. 2018), Japan (Fujimoto et al. 2008), and China (Zhang et al. 2006; Tsou et al. 2012; Wo et al. 2014; Chen et al. 2016; Yu et al. 2016; Lu et al. 2017; Lin et al. 2019; Zhao et al. 2019). Despite these widespread and recurring outbreaks, there is no specific drug or vaccine approved for use against HAdV-3 infections.
The protein capsid of adenovirus comprises 240 hexon trimers and 12 penton bases and fibers, of which the hexon protein contains the major neutralizing epitope ("epsilon") (Crawford-Miksza and Schnurr 1996; Singh et al. 2015). Each hexon monomer is encoded by approximately 900 amino acid residues that form a base (Rux et al. 2003), which is relatively sequence-conserved, and three tower domains that occur as loops lying on the exterior surface of the virion (Rux and Burnett 2000). These loops contain several hypervariable regions, noted as HVR1–HVR6 in loop 1 and HVR7 in loop 2 (Cheng et al. 2018), that are noted collectively as the epsilon epitope. This epsilon epitope provokes the host immune system into producing adenovirus serotype-specific antibodies that are highly effective and long-lasting (Rux et al. 2003; Russell et al. 2006; Pichla-Gollon et al. 2007; Radin et al. 2014; Tian et al. 2015).
Paradoxically, HAdV vectors have been widely and safely used in gene therapy protocols (Stecher et al. 2003; Holterman et al. 2004; Stone et al. 2005; Toth and Wold 2010; Wold and Toth 2013; Duffy et al. 2017). Several drugs incorporating adenoviruses as delivery vectors have been clinically approved and used in patients successfully (Liu and Kirn 2008; Räty et al. 2008). Additionally, HAdVs are the vector foundations of vaccines developed against many pathogens, including Ebola virus (Milligan et al. 2016; Shukarev et al. 2017), HIV (Schooley et al. 2010; Baden et al. 2014; Gu et al. 2014), avian influenza virus (Peters et al. 2013; Scallan et al. 2013), and foot-and-mouth disease virus (Pena et al. 2008), as well as highly contagious bacteria associated with diseases, e.g., tuberculosis (Smaill et al. 2013).
In this study, the commercially-available and gene therapy use approved replication-defective HAdV-5 vector was used to construct a recombinant attenuated human adenovirus type 3 vaccine (Ginn et al. 2018). The complete hexon gene of HAdV-3 GZ01 was cloned into the AdEasyTM Adenoviral Vector, and this type-specific antigen was expressed when the recombinant adenovirus vaccine was inoculated into mice. This complete hexon protein with native conformation in theory was expressed successfully and neutralizing antibodies were produced. The recombinant vaccine is expected to be used in the prevention of ARD outbreaks caused by HAdV-3 infections, and to serve as a model using adenovirus vectors for the construction of other vaccines against additional important serotypes of adenoviral respiratory pathogens.
-
Replication-deficient adenoviruses were cultured in AD293 cells (purchased from Stratagene; CA, USA), which were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 100 IU penicillin/mL, 100 μg streptomycin/mL, and 10% (v/v) fetal calf serum. The HAdV-3 GZ01 strain (accession number DQ099432) was originally isolated from a child with ARD (Zhang et al. 2006) and cultured in A549 cells. Viral genomic DNA was extracted by our modified protease K digestion method.
-
E. coli strains DH5α and BJ5183 are laboratory stocks. Plasmids pShuttle and pAdEasy were purchased from AdEasyTM Adenoviral Vector System (Stratagene; CA, USA). Platinum® Taq DNA polymerase with high-fidelity and restriction enzymes were purchased from Life Technologies Corporation (Carlsbad, CA, USA) and New England Biolabs (MA, USA), respectively.
-
In brief, the complete hexon gene of HAdV-3 GZ01 was cloned into pShuttle and recovered as recombinant psAd3H. This pShuttle vector was then subjected to homologous recombination with pAdEasy, using the highly efficient homologous recombination system in E. coli BJ5183 according to the manufacturer's protocol. Finally, the rAd3H recombinant virus was rescued by transfection with prAd3H in AD293 cells and cultured for 20 generations. This construction strategy is presented as Fig. 1, with the detailed steps as follows.
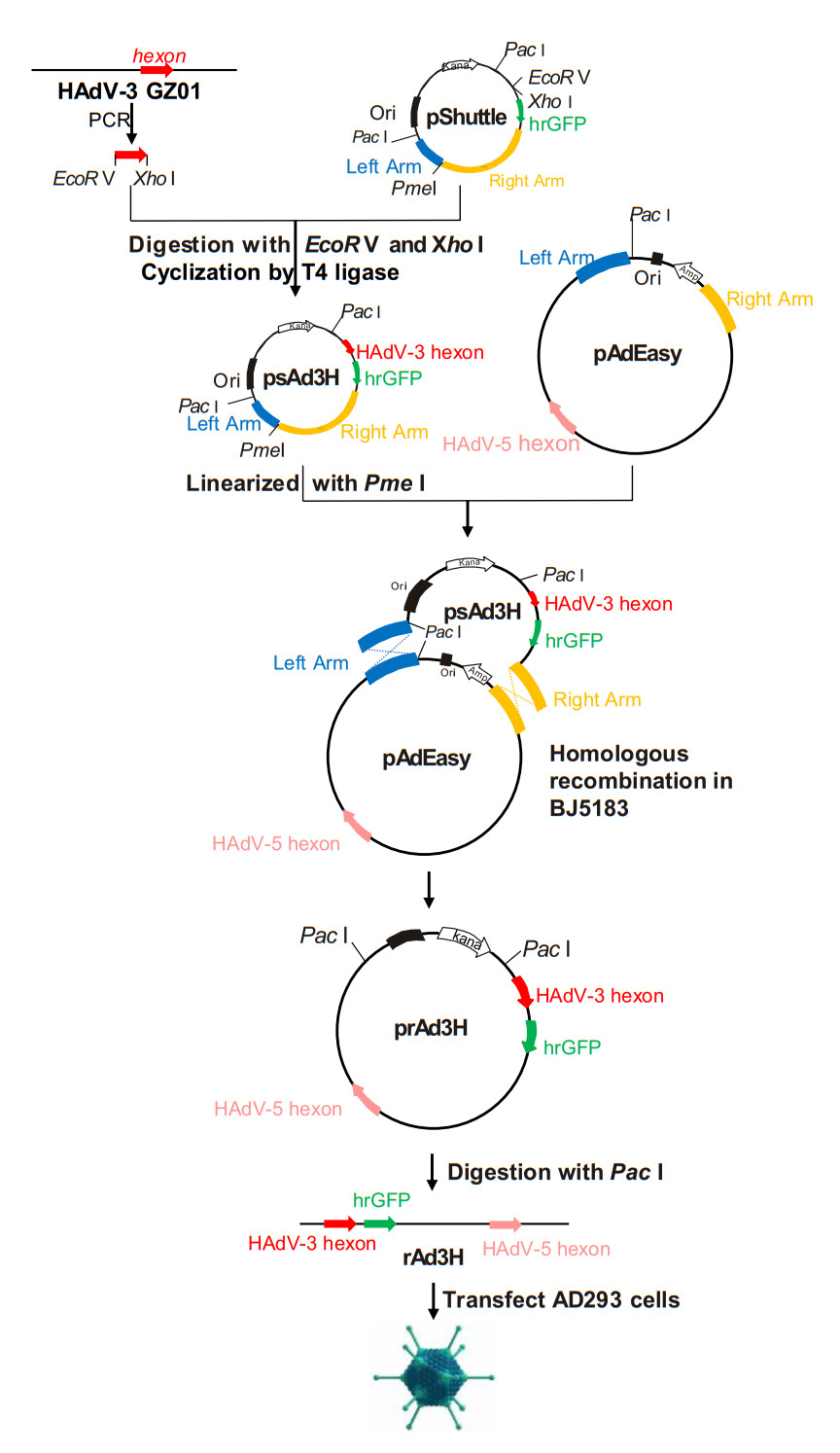
Figure 1. Construction of recombinant adenovirus vector prAd3H and the rescue of recombinant adenovirus rAd3H. The complete hexon gene from HAdV-3 was PCR-amplified and cloned into pShuttle vector. The resultant plasmid psAd3H and pAdEasy were ligated by means of homologous recombination and produced prAd3H, which was further linearized and transfected into AD293 cells. After continuous culture and passages, the recombinant vaccine strain rAd3H was rescued successfully.
First, Platinum® taq DNA polymerase with high-fidelity was used to PCR amplify the complete hexon gene of HAdV-3 GZ01, using the primer pairs Ad3-GZ01-EcoR V-HexF (5′-TGAGATATCGCCACCATGGCCACCCCATC-3′) and Ad3-GZ01-HexR-Xho I (5′-AGACTCGAGTTATGTGGTGGCGTTGCC-3′). Following, two restriction sites, EcoR V and Xho I, were added onto opposite ends of this amplified hexon gene and the PCR products were purified by PCR Clean up Kit (Axygen; New York, USA). Both the PCR products and the pShuttle plasmid were digested by EcoR V and Xho I in 37 ℃, respectively, permitted to reanneal, and then ligated using T4 DNA Ligase at 16 ℃ for 8 h. After ligation, the recombinant was transformed into 200 μL E. coli DH5α competent cells. In the next 12–16 h, the cells were grown in LB agar plates, selected by ampicillin. After selection and amplification of each single clone, the plasmid was extracted and verified by PCR and sequencing, and archived as psAd3H.
Second, approximately 100 ng of pAdEasy and 700 ng of psAd3H linearized with Pme I both were transformed into E. coli BJ5183 competent cells which contained recombinase, and then selected on LB-Kanamycin agar plates. As the small colonies were potential candidate recombination clones (Zhang et al. 2009), they were selected and further identified by PCR screening, using HAdV-3 type-specific primers (Ad3F 5′-AAGACATTACCACTACTGAAGGAGAAGAA-3′ and Ad3R 5′-CGCTAAAGCTCCTGCAACAGCAT-3′) (Han et al. 2013) and producing a PCR product of approximately 300 bp. This PCR-positive plasmid DNA was then amplified in DH5α cells, and additionally identified by sequencing and restriction enzyme digestion (EcoR I, EcoR V, Not I, Sal I, and Xho I). Plasmid Miniprep Kit (Axygen; New York, USA) was used to purify this recombinant plasmid prAd3H.
Third, the prAd3H plasmid was digested with Pac I to release the linear adenovirus genomic DNA, which was then transfected into AD293 cells using Lipofectamine LTX (Life Technologies Corporation; Carlsbad, CA, USA). The cells were examined daily for 10 days for evidence of increased fluorescence and cytopathic effect, which signaled successful transfection and packaging. After 10 days, the cultures were frozen and thawed thrice and then centrifuged at 12, 000 ×g for 10 min. The supernatant was used to infect the AD293 cells. Cells were cultured for about 7 days. Fluorescence and cytopathic effect were monitored each day. The recombinant adenovirus rAd3H was rescued successfully when both GFP and CPE were observed.
-
The transcription of HAdV-3 hexon gene of rAd3H was confirmed by RT-PCR analysis as follows: Total RNA was extracted by RNAiso (TAKARA; Tokyo, Japan) and reverse transcribed into cDNA using the primer HVRR (5′-TTTCTGAAGTTCCACTCGTAGGTGTA-3′). This resultant cDNA was used as the template in a PCR amplification with the primer pairs, HVRF1 (5′-CAGGATGCTTCGGAGTACCTGAG-3′) and HVRR (Zhang et al. 2020). The PCR product was identified using agarose gel electrophoresis and DL10000 markers. Subsequent Western blot analysis confirmed expression of the hexon protein. A culture of rAd3H was frozen and thawed three times, centrifuged at 1000 ×g for 10 min, and the supernatant was collected. The samples were added to loading buffer, boiled for 10 min, electrophoresed in 5% concentrated gel, and 10% Tris-Tricine SDS-PAGE. After PAGE, the proteins were transferred onto nitrocellulose membrane by electrophoresis at 25 V for 40 min. The membrane was probed first by binding with mouse anti-HAdV-3-hexon antibody (Guangzhou HuYanSuo Medical Technology; Guangzhou, China) as the epitope-specific antibody (1:1000 dilution), and followed with goat anti-mouse IgG-HRP (Bioworld; Georgia, USA) as the secondary amplifying antibody. Luminol and hydrogen peroxide were used as chromogenic substrates to score the fluorescence signal.
-
Indirect immunofluorescence assay was performed to detect the virus titer of HAdV-3. 2 × 104 AD293 cells were seeded into each well of a 96-well plate. After an 18–24-h culture, the cells were infected with 100 μL HAdV-3. At 48 h post infection, the cells were fixed in methanol, precooled in -20 ℃, and incubated at 37 ℃ for 30 min with 1% BSA. The mouse anti-HAdV-3-hexon monoclonal antibody, at a dilution of 1:1000 in PBST, was added to wells and incubated at 37 ℃ for an hour. FITC-conjugated goat anti-mouse IgG (1:10000) was then added and the mixture incubated at 37 ℃ for an hour. The number of green fluorescence cells were counted under reversed fluorescence microscopy and virus titer were calculated by fluorescence forming unit (FFU) with the formula of FFU/mL = 10n × average number of GFP positive cells/well × 10 (n: dilution times).
-
AD293 cells infected with rAd3H viruses were grown and harvested when 90% CPE was observed. These cells were frozen and thawed three times, centrifuged at 12, 000 × g for 10 min. Supernatant containing viral particles was collected, upon which a grid covered with carbon support formvar film was floated, for 2 min. This was stained with 10 μL sodium phosphor-tungstate, dried at 40 ℃ for 10 min, and the vaccine strained rAd3H was observed using a TEM technique (FEI TecnaiTM electron microscope).
-
AD293 cells were seeded into 6-well plates with 5 × 105 cells per well. After 16–24 h, the cells were infected with 5 × 104 DNA copies of rAd3H, rAd5 or HAdV-3 strain GZ01, respectively. Cultures were harvested after 12, 24, 36, 48, 72, 84, 96, 108, or 120 h post-infection. These cultures were frozen and thawed three times, then the supernatant was collected after centrifugation. The viral DNA copies at all the time-points were quantified by real-time PCR using the primers pentonF (5′-ACCACCGTCAGTGAAAACG-3′) and pentonR (5′-TATGCCCAGKGCCTTGT-3′) to quantify the genomic DNA copy number. Using HAdV-5 DNA as the template and pentonF and pentonR as primers, the PCR product was ligated into the pMD18-T vector (TAKARA; Tokyo, Japan). The resultant plasmid was used as the standard for real-time PCR quantification. The viral stability of the recombinant vaccine strain rAd3H after continuous passages was further tested. Briefly, the virus was cultured in AD293 cells and passaged for more than 20 generations. At every five generations, adenovirus genomic DNA was extracted; these served as templates for the PCR amplification of HAdV-3 hexon gene, which was then further characterized by DNA sequencing. The 20th generation of rAd3H harvested from the AD293 cells culture was further inoculated into A549 cells and re-cultured for 7 days to determine if there were replication-competent mutants resulting from the continuous passages of rAd3H in AD293 cells. If no green fluorescence was observed during the culturing in A549 cells, it meant that no replication-competent mutants occurred. Therefore, the stability of the recombinant vaccine strain was verified.
-
Discontinuous density-gradient centrifugation was used to purify and concentrate the recombinant adenovirus rAd3H as follows: 6 mL 1.2 g/mL CsCl was first added into the centrifuge tube with 8 mL 1.4 g/mL CsCl slowly added to the bottom subsequently, resulting in a stratification. On the top of the CsCl, 20 mL of the virus culture was then carefully layered, and the tube was centrifuged at 141, 000 ×g at 4 ℃ for 3 h. The virus band was then extracted and transferred to a dialysis bag (MWCO: 14, 000 Da) and dialyzed in a buffer at 4 ℃, with two buffer changes, to remove the CsCl (10 mmol/L Tris–Cl pH 8.0, 2 mmol/L MgCl2, and 5% sucrose). Purified virus was titrated by a modified fluorescence-forming unit assay (Philipson 1961). Briefly, AD293 cells were infected with ten-fold diluted rAd3H virus in 96 well-plates. After 48 h of incubation, rAd3H infected cells are directly observed under fluorescent microscopy. Wells with suitable virus dilution were selected for counting the number of fluorescent cells. The virus titer of FFU was calculated as described above.
-
Four to six-week-old female specific-pathogen-free BALB/c mice were purchased from the Laboratory Animal Center of Southern Medical University (Guangzhou, China) and kept in individual ventilated cage system. Six mice in each group were either inoculated with 1.8 × 108 FFU/kg of HAdV-3 GZ01 or immunized with the rAd3H recombinant vaccine by the intranasal route or intramuscular route, respectively. The negative control group was inoculated with PBS of the same volume. The mice were boosted with the same virus or vaccine strain at day 14 post inoculation. At days 21, 28, 35 and 42, sera from three mice in each group were collected and the 50% neutralizing antibody titer was determined by microculture neutralization test.
-
A microculture neutralization test was performed to determine the HAdV-3 neutralizing antibody titers using a previously described protocol (Zhang et al. 2009). Sera from mice immunized with rAd3H by either intramuscular injection or intranasal inoculation were tested for the presence of HAdV-3 neutralizing antibody. The 50% virus neutralization titers were calculated by Reed-Muench method.
Cells, Virus Strains and Viral Genome
Bacteria, Plasmids and Enzymes
Construction of rAd3H Recombinant Adenovirus Containing the Hexon Gene of HAdV-3
Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Western Blot Analysis
Indirect Immunofluorescence Assay
Transmission Electron Microscopy (TEM) Observation
Adenovirus Growth Characteristics and Stability Assay
Virus Purification and Concentration
Animal Immunization
Microculture Neutralization Test
-
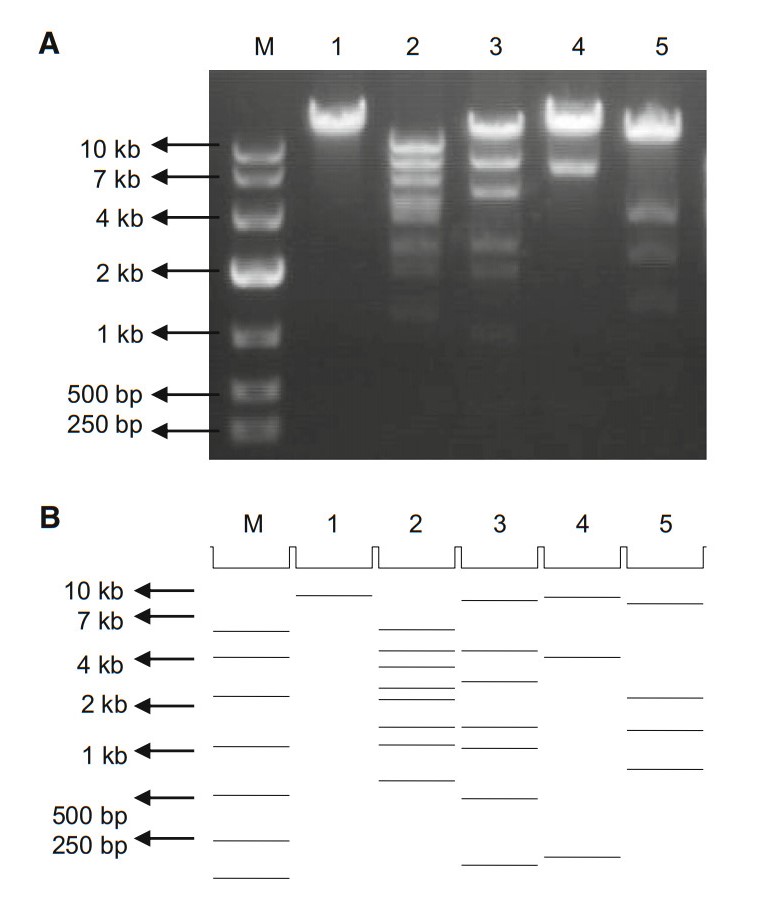
A pShuttle plasmid psAd3H containing the complete hexon gene, which contains the epsilon epitope of HAdV-3, was constructed. This construct also contained the left and right homologous regions that enable homologous recombination with pAdEasy to produce infectious clone prAd3H (Fig. 1). The resultant plasmid was confirmed by DNA sequencing, which not only provided confirmation of the complete hexon gene sequence but also verified that no mutations were introduced. Prior to sequencing, as a rapid check, restriction endonuclease analysis (REA) was performed to characterize the recombinant plasmid rapidly. Restriction maps generated with five restriction endonucleases were consistent with the in silico restriction maps predicted by the Vector NTI 11.5.1 software (Invitrogen Corp; San Diego, CA, USA) (Zhao et al. 2014; Yu et al. 2016; Zhang et al. 2017; Pan et al. 2018) (Fig. 2).
-
Recombinant plasmid prAd3H was linearized by Pac I and transfected into AD293 cells to rescue the recombinant adenovirus rAd3H. Green fluorescence was observed under fluorescence microscopy and the fluorescence density was noted to increase each subsequent day, an indication that the recombinant adenovirus was replicating. At day 10 post-transfection, the virus culture was frozen and thawed three times; after centrifugation, the supernatant was inoculated into AD293 cells. Green fluorescence and CPE were observed and recorded at day 8 post-infection (Fig. 3). The increased fluorescence and CPE suggested that the recombinant virus rAd3H was rescued successfully from AD293 cells.
-
The transcription of the rAd3H hexon gene was confirmed by RT-PCR. A rAd5 virus that containing adenovirus type 5 genome except for E1 and E3 regions was used as a positive control. Viral RNA was extracted from a rAd3H culture and digested with DNase. After digestion, the presumed viral RNA was used as a PCR template for amplifying the hexon gene as a check for any residual DNA. No PCR products were found from these samples, which indicated that the extracted RNA did not contain viral genomic DNA (Fig. 4A). After reverse transcription, the cDNA was used as a PCR template to validate the insert. The resultant amplification of the hexon gene confirmed the rAd3H recombinant adenoviruses. (Fig. 4B).
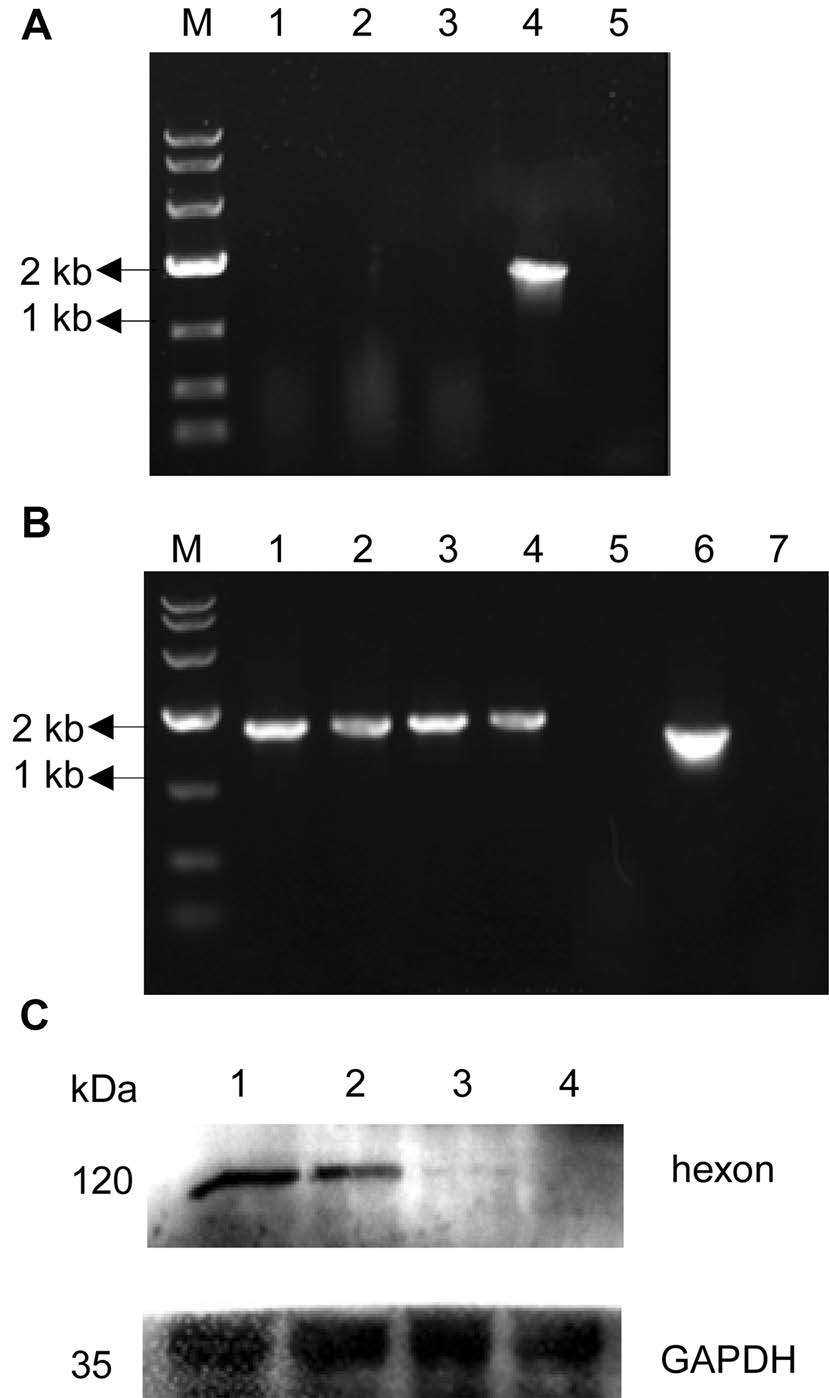
Figure 4. RT-PCR analysis of rAd3H hexon transcription (A, B) and Western blot confirmation of the HAdV-3 hexon protein expression from rAd3H (C). A RNA as template for PCR. Lanes: (M) DL10000 marker; (1) rAd3H; (2) rAd5; (3) mock; (4) PCR positive control; (5) PCR negative control; B cDNA as template for PCR. (M) DL10000 marker; (1–2) rAd3H; (3–4) rAd5; (5) mock; (6) PCR positive control; (7) PCR negative control; C Lanes: (1–2) rAd3H cell supernatant; (3) negative control, rAd5 cell supernatant; (4) mock, supernatant of AD293 cells.
Expression of the HAdV-3 hexon protein in the culture supernatant was also detected by Western blots (Fig. 4C), in which HAdV-3 specific mouse monoclonal antibody was used to identify HAdV-3 hexon. The rAd5 adenovirus was used as the negative control, as it contained the HAdV-5 hexon gene but not the HAdV-3 hexon gene. The HAdV-3 hexon protein of 108 kDa was confirmed in the rAd3H recombinant vaccine strain while no band was found in the rAd5 and mock samples, indicating that during the culture, the HAdV-3 hexon protein of rAd3H can be expressed successfully.
-
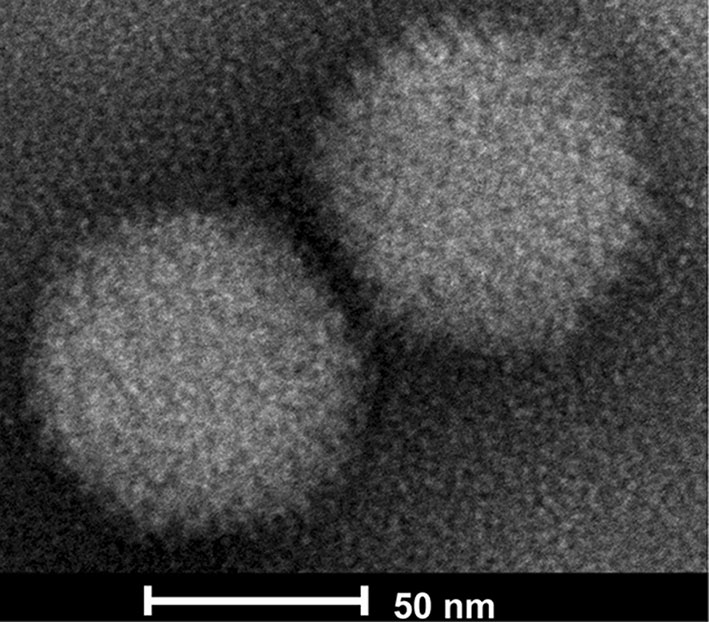
The harvested viral culture from rAd3H was negative-stained by sodium phosphor-tungstate and observed under electron microscopy. Typical adenovirus particles, 70–90 nm in diameter, were clearly visible (Fig. 5). This further confirmed the successful infection and replication of the recombinant HAdV-3 vaccine in AD293 cells.
-
The replication efficiencies of rAd3H, rAd5 and HAdV-3 strain GZ01 were compared by quantification of genomic DNA copies using a real-time PCR method. Primers targeting the conserved region of the penton base gene to represent the genomic DNA number were used in the real-time PCR amplification. The two one-step growth curves of rAd3H and HAdV-3 strain GZ01 showed that the replication efficacy of rAd3H viruses were similar to that of HAdV-3 wild-type strain in AD293 cells, both of which increased similarly during the 60 h post infection. The peaks of DNA copy number of both rAd5 and rAd3H strains were higher than HAdV-3 GZ01 strain, which might be because HAdV-5 genomic DNA is the backbone of both strains and the viruses are cultured in AD293 cells which highly expressed HAdV-5 E1A and E1B genes that promote the HAdV-5 DNA replication (Fig. 6).
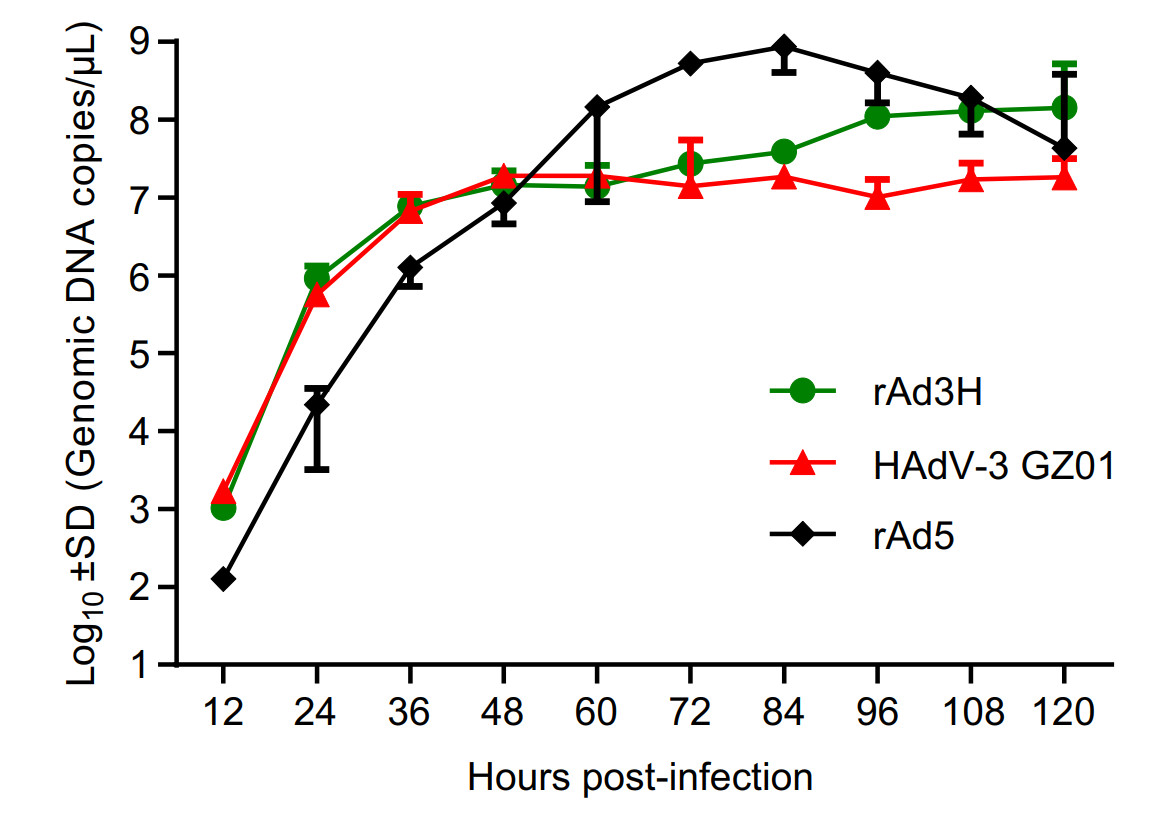
Figure 6. Replication dynamics curves of rAd3H bearing HAdV-3 hexon gene, rAd5 and HAdV-3 wild-type strain GZ01. AD293 cells were infected by the two viruses and harvested at 12, 24, 36, 48, 60, 72, 84, 96, 108, and 120 h post infection. Viral genomic DNA copy numbers in cells and supernatants were determined by real-time PCR for the penton base region.
-
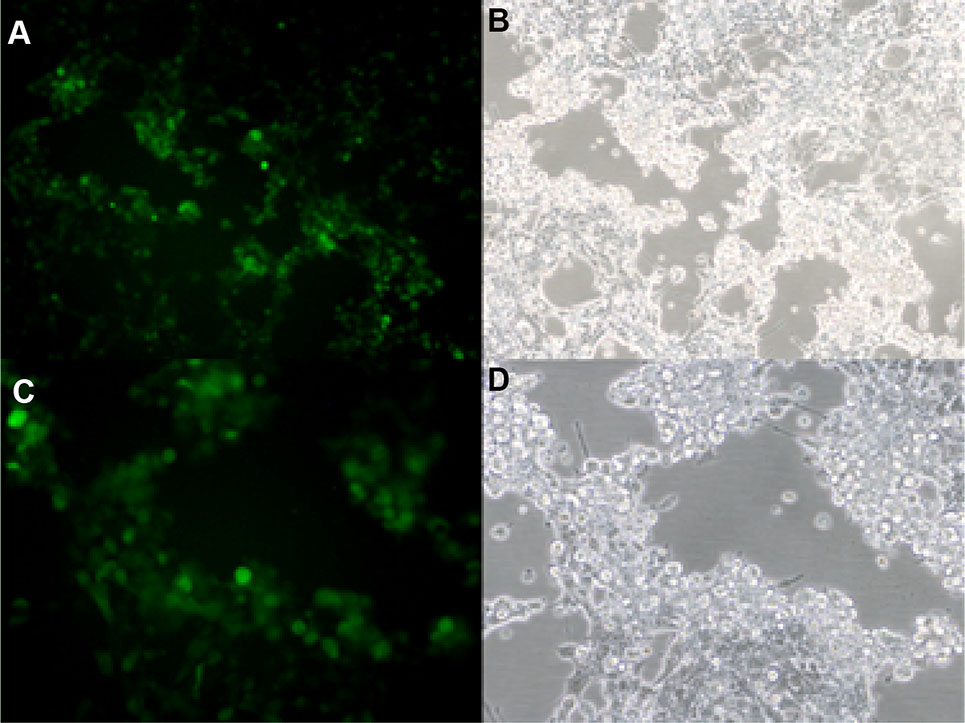
To assess the stability of the insert, recombinant vaccine strain rAd3H was passaged in AD293 cells for at least 20 generations. The virus stability of rAd3H harvested from the 20 generations of culture was verified by PCR amplification and DNA sequencing of the HAdV-3 and HAdV-5 hexon genes. There were no amino acid mutations detected in the HAdV-3 and HAdV-5 hexon gene of rAd3H upon sequence verification. This indicated that both hexon genes in this recombinant vaccine strain are well compatible with each other during the viral replication. As expected, both fluorescence and CPE were observed in the AD293 cells infected by rAd3H from the first to the twentieth generations (Fig. 7A–7D). However, no green fluorescence was detected in the A549 cells infected by rAd3H which was the culture from the first to the twentieth generations of rAd3H in A549 cells (Fig. 7E–7H), which indicated no infectious viral particles produced in A549 cells. PCR amplification of the hexon gene from the virus culture in A549 cells showed no product, which indicated that rAd3H could only replicate in AD293 cells, but not in the A549 cells due to the E1 deletion. No reverse mutations occurred during the continuous passages of rAd3H in AD293 cells. All the results presented confirmed the stability of the vaccine stain rAd3H in AD293 cells (Fig. 7).
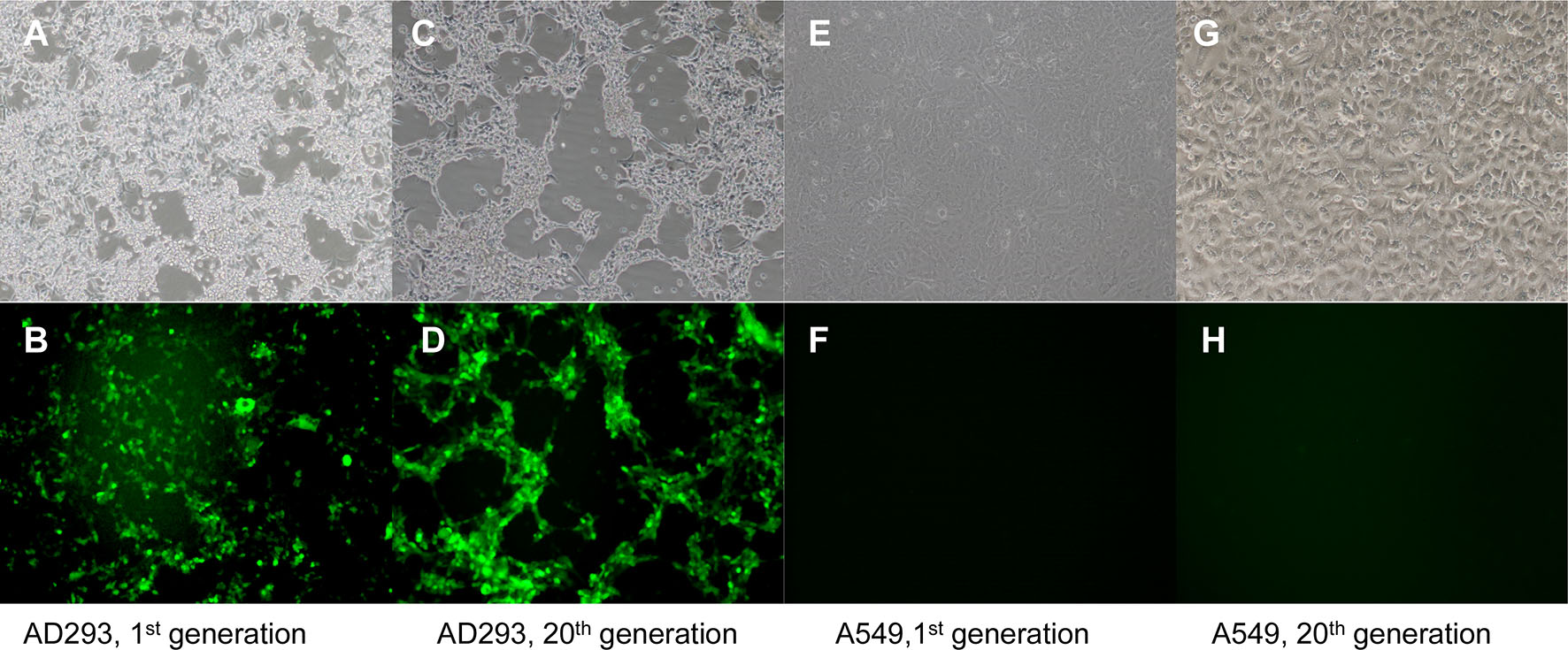
Figure 7. Light and fluorescent microscopy observation of cells infected by the recombinant vaccine rAd3H. A, B AD293 cells infected by the 1st generation of rAd3H; C, D AD293 cells infected by the 20th generation of rAd3H; E, F A549 cells infected by the 1st generation of rAd3H cultured in A549 cells; G, H A549 cells infected by the 20th generation of rAd3H cultured in A549 cells. A, C, E, G are in fluorescence vision; B, D, F, H are in white light vision (100 ×) at day 5 post-infection.
-
Mice were either inoculated with HAdV-3 wild-type strain GZ01 or immunized with the rAd3H recombinant vaccine by either the intranasal route or intramuscular route, respectively, to assess the antibody titer. At day 14 post inoculation/immunization, the mice were boosted with the same inoculant. Mouse sera were collected at day 21, 28, 35, and 42 after the prime inoculation/immunization. The 50% neutralizing antibody titers of mouse sera in the intranasal or intramuscular groups collected at different time points were determined by the microculture neutralization test (Table 1). All of the mice immunized with rAd3H or HAdV-3 GZ01 virus intranasally or intramuscularly produced neutralizing antibodies. However, the mice immunized intramuscularly produced higher and more lasting antibody titers than the intranasally immunized mice. Therefore, apparently the intramuscular immunization was more effective than the intranasal immunization in provoking the immune response. In the wild-type GZ01-innoculated mice, higher neutralizing antibody titers were produced in both intranasal and intramuscular groups, which might be associated with the replication-competence of wild-type adenoviruses as compared with the replication-deficient rAd3H vaccine strain.
Serum collection time rAd3H immunization HAdV-3 wildtype GZ01 inoculation Intranasal Intramuscular Intranasal Intramuscular D21 1:250 1:270 1:1000 1:1428 D28 1:200 1:250 1:1111 1:1000 D35 1:167 1:333 1:1111 1:1667 D42 1:158 1:248 1:916 1:1024 The mice were either immunized with the rAd3H recombinant vaccine or inoculated with HAdV-3 wildtype strain GZ01 intranasally or intramuscularly. At day 21, 28, 35, and 42 after the prime inoculation/immunization, the neutralizing antibodies against HAdV-3 were titrated. Table 1. Determination of the 50% neutralizing antibody titer in mouse sera by the microculture neutralization test.
Construction and Screening of Recombinant prAd3H Plasmid Inserted by the Hexon Gene of HAdV-3
Rescue of Recombinant Adenovirus rAd3H Expressing HAdV-3 Hexon Gene
RT-PCR and Western Blot Assay to Identify the Transcription and Expression of HAdV-3 Hexon Protein
Electron Microscopic Observation of the Vaccine Strain rAd3H
Growth Characteristics of Recombinant Vaccine rAd3H
Stability of Recombinant Vaccine Strain rAd3H
Serum Antibody Titer in Immunized Mice
-
Human adenoviruses are highly contagious pathogens that are associated with several severe and fatal diseases including ARD (Jing et al. 2019; Zhang et al. 2019). ARD associated with HAdV-3 often results in severe morbidity and some fatalities. Additionally, among all of the adenovirus types causing ARD, HAdV-3 appears to be the most common found in pediatrics cases (Chang et al. 2008). There is no vaccine currently available for preventing HAdV-3 outbreaks. In fact, the only two vaccines available against HAdVs (types 4 and 7) are not available to the public. In this study, we provide a novel strategy for the construction of a candidate adenovirus type 3 vaccine that may serve as a template for developing similar vaccines for use against other pathogen HAdVs. The time-tested and safe HAdV-5 replication-deficient vaccine vector was used for the construction of this candidate vaccine strain that harbors the immunogenic epitope from human adenovirus type 3 hexon. We demonstrate that the administration of this recombinant and replication-deficient rAd3H strain could elicit the significant increase of neutralizing antibodies against HAdV-3; therefore, it could be considered a candidate vaccine strain to be used for the prevention of HAdV-3 infection or epidemics.
There are three methods used to construct an adenovirus vector: in vitro ligation, Cre/loxP system, homologous recombination in eukaryotic cells or in prokaryote cells method. The genome of human adenoviruses is about 35 kb. Compared with the other methods, the method of homologous recombination in prokaryote cells has the advantages of a simpler protocol, higher recombination efficiency, and easier screening of positive clones. In this study, we used the homologous recombination in E. coli BJ5183 to construct a recombinant adenovirus vector to serve as the basis for a vaccine against a commonly circulating adenoviral respiratory pathogen. This recombinant DNA vector construction protocol consists of two vectors: an adenovirus backbone plasmid, pAdEasy, and a pShuttle plasmid. The pShuttle plasmid contains a multiple cloning site and a kanamycin resistance gene, which support the insertion and maintenance of a foreign gene, as well as provides for the rapid screening for recombination. A PCR-derived product comprising the complete hexon gene (epsilon epitope) of adenovirus type 3 was directly cloned into the multiple cloning site of pShuttle using the EcoR V and Xho I restriction sites. The linearized psAd3H and pAdEasy were transformed into an engineered bacterial strain BJ5183 that was recA proficient and supplied the machinery necessary to execute the recombination event. Homologous recombination was carried out to obtain the vaccine vector bearing the complete HAdV-3 hexon gene. To overcome the undue homologous recombination during the bacterium culture, the recombinant time in BJ5183 was restricted to no more than 8 h, then recombinant bacterial clones were screened by kanamycin resistance. Following the construction of the recombinant plasmid, the putative size of the insert allows for an initial screen, with additional identification, confirmation, and characterization by PCR, REA, DNA sequencing, and other assays.
The rAd3H vaccine contains most genes of HAdV-5 except the E1 and E3 regions, as well as the complete HAdV-3 hexon gene. The E1 region is essential for the assembly of infectious virus particles, so the replication of rAd3H can only take place in adenovirus packaging cell line like AD293 cells which express the E1 proteins of HAdV-5, while rAd3H cannot replicate in any kind of human cells. Therefore, the safety of rAd3H recombinant adenovirus vaccine is guaranteed.
The recombinant vaccine rAd3H expresses the complete HAdV-3 hexon gene in infected cells, which can maintain the native conformation of the whole HAdV-3 hexon protein. In theory, this will guarantee a better immune effect. However, mice immunized with recombinant vaccine rAd3H produced lower neutralizing antibody titers than wild-type HAdV-3 strain. One of the causes may be that the capsid proteins of fiber and penton base in the wild-type viruses also provoke certain immunogenicity (Gahery-Segard et al. 1998; Feng et al. 2018). In rAd3H vaccine strain, no HAdV-3 fiber and penton protein were expressed, while there was in HAdV-3 wild-type strain. Another cause is the rAd3H vaccine strain is replication deficient, which cannot replicate and express proteins well in normal cells. As the result, the induced humoral immunity by the wild-type strain in mice was stronger than replication deficient vaccine strain.
In summary, a recombinant and attenuated adenovirus vaccine candidate against HAdV-3 was constructed based on a commercially-available, replication-defective HAdV-5 vector that has been widely used in previous gene therapy protocols and vaccine development. This potential vaccine candidate had a similar replicative efficacy in AD293 cells as the wild-type HAdV-3 strain. The recombinant is also highly stable and could elicit significant neutralizing antibodies against HAdV-3, with both intranasal and intramuscular inoculation of mice. Therefore, this recombinant adenovirus vaccine is a promising vaccine candidate against HAdV-3. The strategy of using a clinically approved, previously used in gene therapy settings, and replication-defective HAdV-5 vector as the basis of the vaccine provides a novel approach for future development of additional adenovirus vaccine candidates against all of the other adenoviral genotypes causing ARDs and other diseases.
-
This work was supported by Grants from the National Key Research and Development Program of China (2018YFE0204503), National Natural Science Foundation of China (31570155, 31370199), and Natural Science Foundation of Guangdong Province (2018B030312010), as well as the Guangzhou Healthcare Collaborative Innovation Major Project (201803040004, 201803040007).
-
QZ designed the experiments. YY, SJ and LF carried out the experiments. YY, SJ, LF, JZ, ZZ, ML, SZ, JO, WL, WG, XW, JW, DS and QZ analyzed the data. YY, DS, and QZ wrote the paper and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
The whole study was approved by the Southern Medical University Animal Ethics Committee. All institutional and national guidelines for the care and use of laboratory animals were followed.














 DownLoad:
DownLoad: