HTML
-
Zika virus belongs to the genus Flavivirus in the family Flaviviridae, which also includes the dengue, West Nile, and Japanese encephalitis viruses (Haddow et al. 2012). Zika virus is a newly emerging neurotropic flavivirus and was first discovered in the Zika forest in Uganda in 1947 (Dick 1952). Most patients infected with Zika virus appear asymptomatic or develop an inconspicuous febrile disease. However, owing to the neurotropic nature of Zika virus, its infection may cause severe neurological diseases, including Guillain-Barré syndrome and congenital microcephaly (Duffy et al. 2009; Fauci and Morens 2016). In recent years, neonatal microcephaly has caused increasing concern. Thus, infection with Zika virus has become a global public health issue. Currently, researchers are focusing on virulence proteins related to the spread, infectivity, and replication of Zika virus in hopes of developing vaccines or antiviral drugs that can be used as a preventative or therapeutic agent.
Researchers first extracted the amide alkaloid PL from the medicinal plant Piper longum L. in 1961 and determined its molecular structure in 1984 (Boll et al. 1984). In recent years, PL was found to have various biological activities, such as antiplatelet, anxiolytic, neuroprotective, anti-arteriosclerosis, antibacterial, and, most importantly, anticancer activities (Bezerra et al. 2013). The antitumor activity of PL has been studied in numerous cancer models. The results revealed its cytotoxicity and inhibitory effect on the growth of various cancer cell lines, such as colon, lung, breast, pancreas, kidney, and prostate cancers. The antitumor properties of PL are generally related to the inhibition of glutathione S-transferase P and carbonyl reductase 1. This impairs redox homeostasis and reactive oxygen species (ROS) production in tumor cells (Bezerra et al. 2007; Raj et al. 2011; Chen et al. 2015; Duan et al. 2016).
Heme oxygenase-1 (HO-1) is an enzyme that catalyzes the rate-limiting step of heme degradation to carbon monoxide, diacetylcholine, and free iron (Raimotenhunen et al. 1968). HO-1 expression is induced by its heme substrates, and various non-heme inducers, such as heat shock proteins, inflammatory cytokines, endotoxin, and ROS, suggesting that HO-1 may play a key role in the maintenance of cell homeostasis (Maines 1988; Applegate et al. 1991). In addition, researchers have shown that the upregulation of HO-1 can limit infections from hepatitis B and C viruses, Ebola virus, human immunodeficiency virus, and dengue virus (Devadas and Dhawan 2006; Protzer et al. 2007; Lehmann et al. 2010; Hill-Batorski et al. 2013; Chen et al. 2018b). Studies have also shown that the induction of HO-1 expression by heme has significant anti-Zika virus properties (Huang et al. 2017).
Nrf2 is a nuclear transcription factor in the cap-'n'- collar (CNC) family with a molecular weight of 66 kDa. It contains a highly conserved basic region leucine zipper structure. Nrf2 is an important nuclear factor that activates the transcription of HO-1 (Itoh et al. 1999). DC-SIGN, Axl, Tyro3, and TIM1 promote the entry of Zika virus into cells. Among these factors, Axl plays a major role (Hamel et al. 2015). Studies of primary human neural and placental cells have shown a correlation between Axl expression and Zika virus infection (Nowakowski et al. 2016; Tabata et al. 2016). However, another report proposed that Axl was unlikely to act as a receptor for the entry of Zika virus, but may instead promote the infection of Zika virus in human astrocytes by antagonizing type I interferon signaling (Chen et al. 2018).
Thus far, there have been no reports of an effective method for the prevention or treatment of Zika virus infection. However, some studies have mentioned that the United States Food and Drug Administration-approved drug Panhematin®, which contains the active ingredient hemin, can induce HO-1 expression and inhibit Zika virus infection, though the mechanism underlying its action remains unclear (Huang et al. 2017). The current study is based on the following three points: (1) PL is effectively and selectively killing of many tumor cells by increasing ROS level; (2) the increase in ROS level leads to oxidative stress that upregulates HO-1; and (3) HO-1 inhibits the replication of Zika virus. We hypothesized and verified that PL increased the level of ROS in tumor cells, causing cellular oxidative stress. This induced the expression of HO-1, thereby inhibiting the replication of Zika virus.
-
The cells used in this study were human brain microvascular endothelial cells (HBMECs) donated by Sheng-He Huang (University of Southern California, Los Angeles, CA, USA), and Vero and human umbilical vein endothelial cells (HUVECs), which were originally held in our laboratory. HBMECs were cultured in RPMI 1640 medium. Vero and HUVECs were cultured in Dulbecco's modified Eagles medium. Both media contained 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA). All cells were cultured in 5% CO2 at 37 ℃. Zika virus Z16006, donated by the Institute of Virology of Guangdong Provincial Center for Disease Control and Prevention, was conserved and amplified by our laboratory.
-
After the three type of cells were plated in six-well plates, they were further cultured for 24 h, then inoculated with Zika virus Z16006 at a multiplicity of infection (MOI) of 1 and simultaneously treated with 0, 2.5, 5 and 10 μmol/L PL at 37 ℃ for 30 min. The virus solution was removed after inoculation. Next, the cells were rinsed twice with phosphate-buffered saline and PL was added back to the cells and was maintained at a constant concentration. Cells were further incubated at 37 ℃ under 5% CO2 for 12, 24 or 48 h. All conditions were performed in triplicate and repeated a minimum of three times (N = 9).
-
Virus extraction from the cell culture supernatant was performed according to the manufacturer's instructions using a viral RNA extraction kit (Qiagen, Du¨sseldorf, Germany). RNA was solubilized in 60 μL of buffer AVE (Qiagen). RNAiso Plus (TaKaRa Biotechnology, Dalian, China) was used to extract total RNA from HBMECs, HUVECs, and Vero cells. RNA pellets were dissolved in 60 μL of diethylpyrocarbonate solution and stored at - 80 ℃. RNA quantification was performed on a Spark® 10 M microplate reader (Tecan Systems, Männedorf, Switzerland).
Quantitative PCR (qPCR) was performed using an RR047A kit (TaKaRa Biotechnology) according to the manufacturer's instructions. The total volume of 20 μL solution contained no more than 1 lg of total RNA. The procedures included reverse transcription at 37 ℃ for 15 min, 85 ℃ for 5 s, followed by the removal of genomic DNA at 42 ℃ for 2 min. The primers and probe used for qPCR are shown in Supplementary Table S1. qPCR was performed using BestarTM qPCR MasterMix (DBI Bioscience, Ludwigshafen, Germany) and TB GreenTM Premix Ex TaqTM II (TaKaRa Biotechnology). All steps were conducted according to the manufacturer's instructions. qPCR data were analyzed using the 2-ΔΔCt method, and the results were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which was used as an internal control. The IC50 was determined from doseresponse curves. All variables were performed in triplicate and repeated a minimum of three times (N = 9).
-
Experimental (PL treatment), control (cell suspension), and blank (medium supplemented with 10% fetal bovine serum) groups were used. After seeding the cells for 12 h, 200 μL of PL diluted in whole culture medium was added to the experimental group at final concentrations of 0, 5, 10, 20, and 40 μmol/L. After 24 h of incubation with PL, 20 μL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (0.5%) was added to each well, and the culture was continued. After 4 h, the culture solution was discarded, and 100 μL of dimethyl sulfoxide was added to each well. Subsequently, the optical density (OD) at 490 nm of each well was detected using a microplate reader. The relative survival rate (%) of each group was calculated as follows: (experimental group OD value - blank group OD value)/(control group OD value - blank group OD value) × 100. CC50 was the concentration of PL required to inhibit HBMECs, HUVECs, and Vero cell growth by 50% compared with the growth of the untreated cells. The selectivity index (SI) values were calculated as CC50/IC50. The PL concentration with the best SI was selected for all subsequent experiments.
-
Cells seeded into six-well plates were harvested after washing three times with phosphate-buffered saline and then lysed in radioimmunoprecipitation assay lysis buffer containing phenylmethylsulfonyl fluoride (Genshare, Xi'an, China). Proteins were separated by 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis or CFAS anyKD polyacrylamide gel electrophoresis (Genshare) and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked with 3% bovine serum albumin in trisbuffered saline/0.1% Tween 20 for 4–6 h and then incubated with primary antibodies at a 1:5000 dilution overnight at 4 ℃. Anti-rabbit immunoglobulin-horseradish peroxidase conjugates were used as secondary antibodies (dilution 1:10, 000; Bioworld Technology, St. Louis Park, MN, USA). The membranes were developed with BiodlightTM ECL Chemiluminescent HRP Substrate (High Sensitivity) (Bioworld Technology). Protein bands were visualized with a Tanon 6200 Luminescent Imaging Workstation (Tanon Science and Technology, Shanghai, China). Rabbit antibodies against HO-1 and nuclear factor erythroid 2-related factor-2 (Nrf2) were purchased from Abcam (Cambridge, UK). A rabbit antibody against b-actin was purchased from Bioworld Technology. All Western blots are representative of three independent experiments.
-
HBMECs and Vero cells were seeded into six-well culture plates for transfection. After seeding the cells for 12 h, they were incubated with 25 nmol/L nonsense control or HO-1 siRNA for 6–8 h in serum-free Dulbecco's modified Eagles medium and transfected using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer's transfection instructions. The suquence of HO-1 siRNA was CCAAGTTCAAGCAGCTCTA and it was compounded by Guangzhou RiboBio. After 24 h, the cells were then treated with 10 μmol/L PL for 24 h and simultaneously infected with Zika virus at an MOI of 1. Total RNA and protein in the cells were then collected, and the expression of HO-1 mRNA and protein was measured. Transfection efficiency of HO-1 knockdown was assessed by Western blot analysis using a specific HO-1 polyclonal antibody (Abcam) with a rabbit anti-actin polyclonal antibody (Bioworld Technology) as the control. RT-qPCR analysis of Zika virus expression and HO-1 in HBMEC and Vero cell treated with N-acetyl-L-cysteine (NAC) (5 mmol/L) 1 h before treatment with 10 μmol/L PL, and subsequently infected with Zika virus at an MOI of 1 at the same time, and total RNA was isolated and quantified for virus replication by real-time RT-PCR 24 h after infection. Virus infection was quantified by qPCR.
-
ROS level was detected by ROS Detection Kit (KeyGEN BioTECH, Jiangsu, China). 5 × 104 Vero cells were seeded in 24 well cell culture plate. After 24 h, the cells were treated with 5 μmol/L PL, 5 μmol/L PL plus 5 mmol/L NAC, or 5 mmol/L NAC, respectively. The cell culture medium was removed and 350 μL 105 μmol/L DCFH-DA was added and incubated at 37 ℃ for 20 min, after cells were treated with PL. Then, cells were washed with nutrient solution three times to remove DCFH-DA. Cells were collected and ROS were detected with 488 nm excitation wave length and 525 nm emission wave length. The ROS level was normalized with control group.
-
Data from at least three independent experiments are presented as means ± SD and were analyzed using the SPSS 19.0 software (IBM, Chicago, IL, USA). Differences among treatment groups were determined by Student's ttest or one-way analysis of variance with Dunnett's multiple comparison post-hoc test. P values < 0.05 from a two-tailed test indicated statistically significant differences. All conditions were performed in triplicate and repeated a minimum of three times (N = 9).
Cell Lines and Virus
Viral Infection and PL Treatment
RNA Isolation, RNA Reverse Transcription, and Real-Time Polymerase Chain Reaction (PCR)
Cytotoxicity Test of PL on HBMECs, HUVECs and Vero Cells
Western Blot Analyses
Small Interfering (si)RNA Transfections
NAC Treatment and ROS Detection
Statistics
-
HBMECs, HUVECs, and Vero cells were infected with Zika virus (MOI = 1) and simultaneously treated with 0 to 10 μmol/L PL. Following removal of the virus solution, PL was added back to the cells and was maintained at a constant concentration. After 24 h, the total RNA of the cells was extracted. mRNA levels of the Zika virus E gene and the internal control gene, GAPDH, were measured by qPCR. The relative content of Zika virus mRNA was significantly different between the PL-treated and non PLtreated groups (Fig. 1A). In HBMECs, when the PL concentration was 5 and 10 μmol/L, the relative content of Zika virus RNA decreased by 54.2% and 93.3% compared to the control group, respectively. In HUVECs, at 10 μmol/L PL, the relative content of Zika virus mRNA decreased by 23.8% compared to the control group, whereas in Vero cells, at PL concentrations of 2.5, 5, and 10 μmol/L, the relative content of Zika virus mRNA decreased to 31.4%, 68.1%, and 87.6% compared to the control group, respectively (Fig. 1A). Thus, we confirmed that PL inhibited the replication of Zika virus in a concentration-dependent manner.
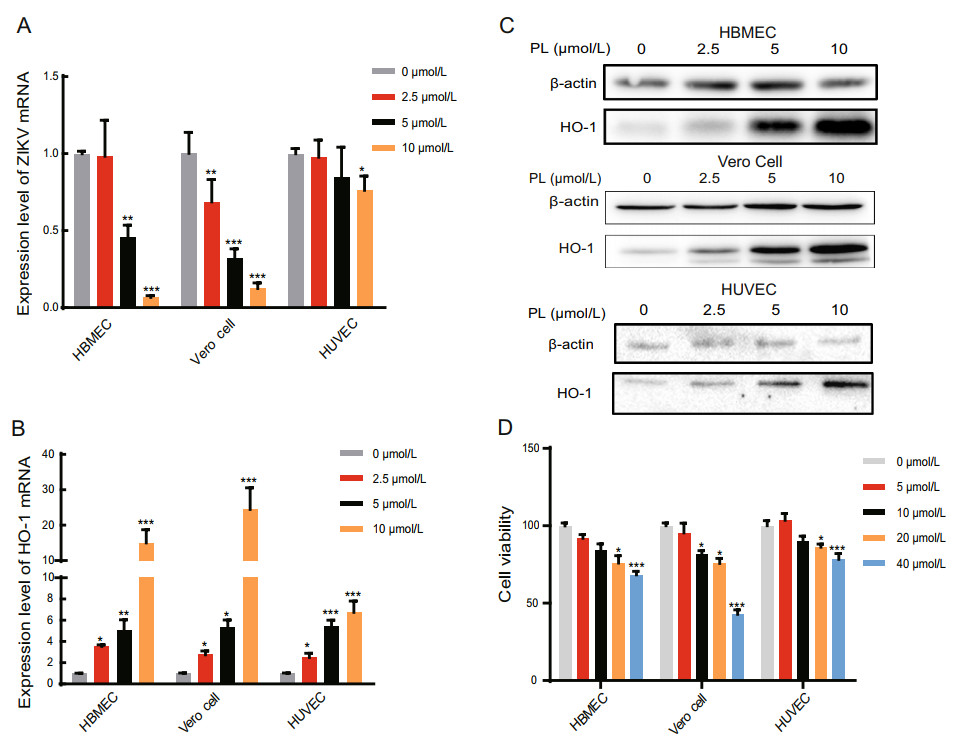
Figure 1. PL reduced Zika virus and increased the HO-1 mRNA level with increasing concentration. A, B RT-qPCR analysis of ZIKV mRNA and HO-1 expression in HBMEC, Vero Cell, and HUVEC at 24 hpi. PL concentration was 0, 2.5, 5 and 10 μmol/L. The duration of PL treatment was 24 h. The three cell types were inoculated with ZIKV at an MOI of 1. The significance of differences between groups was determined using one-way ANOVA. C HO-1 protein expression was analyzed by Western blot using an anti-HO-1 antibody at 24 h post-PL treatment. Antibody against b-actin served as the protein loading control. D Cell viability test using MTT assay to examine the toxicity of cells upon treatment with different concentrations of PL. PL concentration was 0, 5, 10, 20 and 40 μmol/L. Absorbance values were measured at 490 nm. All variables were repeated for at least three times. The significance of differences between groups was determined using one-way ANOVA. *P < 0.05; ** P < 0.01, ***P < 0.001.
We then determined if PL increases HO-1 expression. qPCR and Western blot analysis showed that consistently with our hypothesis, as the PL concentration increased, the relative expression of HO-1 mRNA and protein also gradually increased (Fig. 1B, 1C). In HBMECs, when the PL concentration was 2.5, 5, and 10 μmol/L, the relative HO-1 mRNA expression levels were 3.53, 5.08, and 12.2 times higher than those at 0 μmol/L, respectively. Similarly, in Vero cells, when the PL concentration was 2.5, 5, and 10 μmol/L, the relative HO-1 expression levels were 2.75, 5.31, and 24.5 times higher than those at 0 μmol/L, respectively. Finally, in HUVECs, when the PL concentration was 2.5, 5, and 10 μmol/L, the relative expression was 2.50, 5.42, and 6.76 times higher than that at 0 μmol/L, respectively (P < 0.05 for all comparisons to 0 μmol/L) (Fig. 1B). Taken together, these results demonstrate that PL inhibited Zika virus replication while increasing HO-1 expression in a concentration-dependent manner (linear relationship, P < 0.05).
Furthermore, cytotoxicity results showed that 10 μmol/L PL was slightly toxic to HBMECs and Vero cells, leading to a cell survival rate of 80%. However, it did not exert significant toxicity to HUVECs (Fig. 1D) (P < 0.05). Hence, as shown in Table 1, according to the calculated SI, 10 μmol/L PL was selected for subsequent experiments.
Cell linea CC50(μmol/L)b IC50(μmol/L)c SId HBMEC 111.717±0.226 5.086±0.53 21.966 Vero cell 35.625±0.239 3.615±0.345 9.855 HUVEC 324.804±0.369 22.359±0.429 14.527 aCells treated with PL. bConcentration of PL (μmol/L) that reduced the viability of cells by 50%. cConcentration of PL that reduced the number of Zika virus particles in the cells by 50%. dSelectivity index value. Table 1. Cytotoxic effect, antiviral activity, and selectivity index of PL.
To determine the correlation of inhibition effect of PL with time post infection, HBMECs, Vero cells, and HUVECs, infected with Zika virus, were cultured in the presence and absence of 10 μmol/L PL. Total cellular RNA was collected after 0, 12, and 24 h. qPCR results showed that, as the duration of infection increased, the relative expression of Zika virus mRNA decreased gradually in the PL-treated group compared with that in the control group (Fig. 2B, 2C). Zika virus replication was inhibited by 65.9% (HBMECs), 70.8% (Vero cells) and 25% (HUVECs) compared to untreated cells at 12 h post-infection (hpi) and 77.7% (HBMECs), 86.6% (Vero cells) and 50.1% (HUVECs) compared to untreated at 24 hpi (P < 0.05). However, in HUVECs, Zika virus replication was found to decrease initially, followed by a subsequent increase in cells treated with both 0 and 10 μmol/L PL. This finding may be related to the susceptibility of HUVECs to Zika virus.
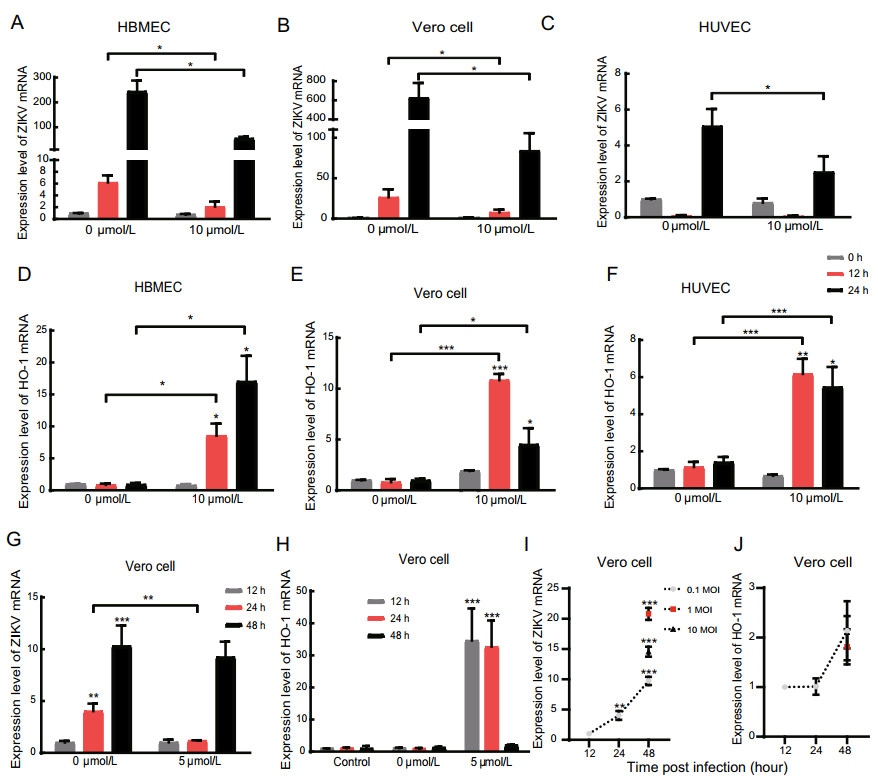
Figure 2. PL reduced Zika virus and increased the HO-1 mRNA level with increasing time. A, B, C RT-qPCR analysis of ZIKV mRNA expression in HBMEC, Vero Cell and HUVEC 0-, 12- and 24-hpi. Three cell types were inoculated with ZIKV at an MOI of 1. D, E, F RT-qPCR analysis of HO-1 expression in HBMEC, Vero Cell and HUVEC 0-, 12- and 24-hpi. G, H RT-qPCR analysis of ZIKV mRNA and HO-1 expression in Vero Cell at an MOI = 0.1 12-, 24- and 48-hpi. The significance of differences between groups was determined using one-way ANOVA, and the significance of differences between the 0 μmol/L and 5 μmol/L or 10 μmol/L was determined using two-tailed Student's t-tests. I, J RT-qPCR analysis of ZIKV mRNA and HO-1 expression in Vero Cell at an MOI = 0.1, 1 and 10 12-, 24-and 48-hpi. *P < 0.05; ** P < 0.01, ***P < 0.001.
Next, the level of HO-1 mRNA was detected by qPCR. Experiment results showed that HO-1 was increased by 8.82-(HBMECs), 12.50-(Vero cells) and 4.35-fold (HUVECs) compared to untreated cells at 12 hpi, and by 17.20-(HBMECs), 3.60-(Vero cells) and 2.86-fold (HUVECs) compared to untreated cells at 24 hpi (P < 0.05) (Fig. 2D, 2E, and 2F). Together, these results demonstrate that PL inhibits the replication of Zika virus and increases HO-1 expression in a time-dependent manner (linear relationship, P < 0.05); however, in Vero cells and HUVECs, the relative expression of HO-1 reached a maximum after 12 h of treatment with PL (Fig. 2E, 2F). When we extended the experiment to 48 h post infection, the level of ZIKV and HO-1 mRNA were reverted to the level which similar to the group without PL (Fig. 2G, 2H). And we also found that the level of HO-1 mRNA had not been affected when Vero cells were infected with ZIKV at MOIs of 0.1, 1 and 10 (Fig. 2I, 2J).
-
To confirm that the PL-induced increase in HO-1 expression inhibited the replication of Zika virus, HBMECs and Vero cells were transfected with HO-1-specific siRNA for 24 h, infected with Zika virus, and then treated with 10 μmol/L PL for 24 h. Total RNA and protein in the cells were then collected, and the expression of HO-1 mRNA and protein was measured (Fig. 3). The results showed that, in HBMECs, the relative expression of Zika virus mRNA was restored partially in the HO-1 siRNA + PL + Zika virus group compared with that in the HO-1 nonsense control (NC) + PL + Zika virus group, though it did not return to the level of the HO-1 siRNA + Zika virus group (Fig. 3A).
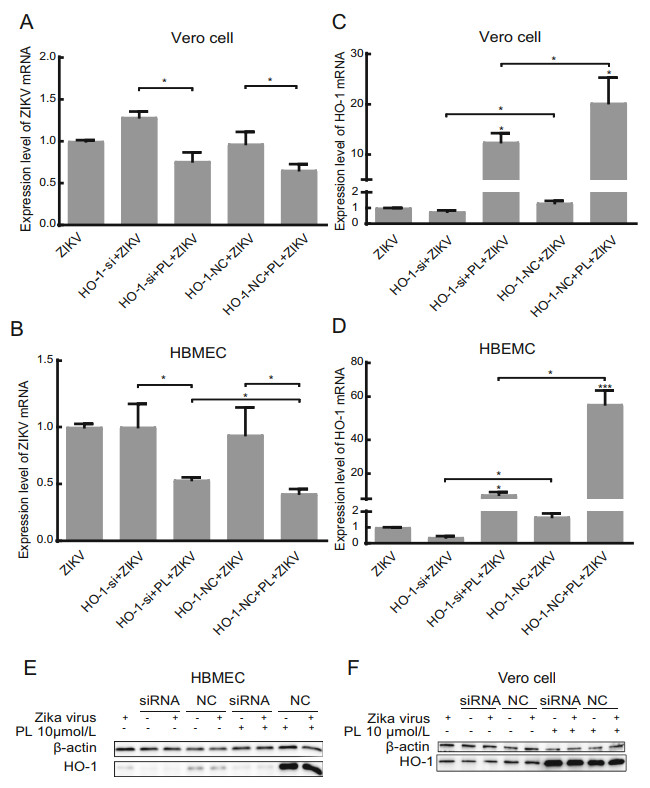
Figure 3. HO-1 inhibits the replication of Zika virus in HBMECs but not in Vero cells. A, B RT-qPCR analysis of ZIKV mRNA and HO-1 expression in HBMEC at 24 hpi. HBMECs were inoculated with ZIKV at an MOI of 1. Differences, compared group ZIKV to others, were represented by #. C, D RTqPCR analysis of ZIKV mRNA and HO-1 expression in Vero cells at 24 hpi. Vero cells were inoculated with ZIKV at an MOI of 1. Differences, compared group ZIKV to others, were represented by #. E, F HO-1 protein expression was analyzed in HBMEC and Vero cell by Western blot. Group of siRNA and NC both had added virus and no virus group. PL concentration was 10 μmol/L. siRNA was HO-1 siRNA, and NC was the negative control. Antibody against b-actin served as the protein loading control. All variables were repeated for at least three times. * P < 0.05, *** P < 0.001.
siRNA against HO-1 mRNA exhibited a significant silencing effect, as shown by the decreased HO-1 expression (Fig. 3B, 3D). The level of HO-1 mRNA in the HO-1 siRNA + PL + Zika virus group was decreased by 86.5% (HBMECs) and 38.5% (Vero cells) compared to that in the HO-1 NC + PL + Zika virus group. However, HO-1 siRNA was not good enough to completely silence the increase in HO-1 induced by PL, and the level of HO-1 mRNA in the HO-1 siRNA + PL + Zika virus group was 20.0 (HBMECs) and 16.2 (Vero cells) times higher than that in the HO-1 siRNA + Zika virus group (P < 0.05) (Fig. 3B, 3D). The finding that the relative expression of Zika virus mRNA in the HO-1 siRNA + PL + Zika virus group did not return to the same level as that in the HO-1 siRNA + Zika virus group suggested that PL may have other mechanisms for inhibiting the replication of Zika virus. However, it appears that in HBMECs PL inhibited the replication of Zika virus by inducing an increase in the expression of HO-1. The Western blot results demonstrated that changes in the HO-1 protein expression level in HBMECs were consistent with those at the mRNA level (Fig. 3E). Similar changes were observed in Vero cells, but there was no significant difference in the relative expression of Zika virus mRNA between the HO-1 siRNA + PL + Zika virus group and the HO-1 NC + PL + Zika virus group (P = 0.671, Fig. 3C). This result may have been attributed to siRNA against HO-1 not exhibiting a strong silencing effect in Vero cells, as we noted that the level of HO-1 mRNA in the HO-1 siRNA + PL + Zika virus group and HO-1 siRNA + Zika virus group were decreased by only 38.5% and 41.9% compared to that in the HO-1 NC + PL + Zika virus group and the HO-1 NC + Zika virus group. Furthermore, the Western blot results did not show a significant reduction in the HO-1 expression level (Fig. 3F).
-
Based on the published literature, ROS are one of the factors that induce HO-1 expression (Loboda et al. 2016). Thus, we hypothesized that HO-1-mediated protection against Zika virus infection involves ROS and Nrf2. We pretreated cells for 1 h with a ROS scavenger, N-acetyl-Lcysteine (NAC), and then infected the cells with Zika virus and treated them with PL at the same time. As shown in Fig. 4A and 4B, in HBMECs and Vero cells, the expression levels of HO-1 mRNA in the NAC + PL + Zika virus groups were lower than that in the PL + Zika virus groups, whereas the relative expression of Zika virus in the NAC + PL + Zika virus groups were higher than that in the PL + Zika virus groups. The difference in Vero cells (P < 0.05), but not in HBMECs (P = 0.25), was statistically significant. The ROS level and Western blotting results were consistent with the qPCR data (Fig. 4C, 4D). The protein bands showed that NAC completely eliminated intrinsic HO-1 in the cells, but after treatment with PL, HO-1 was not completely cleared by NAC.
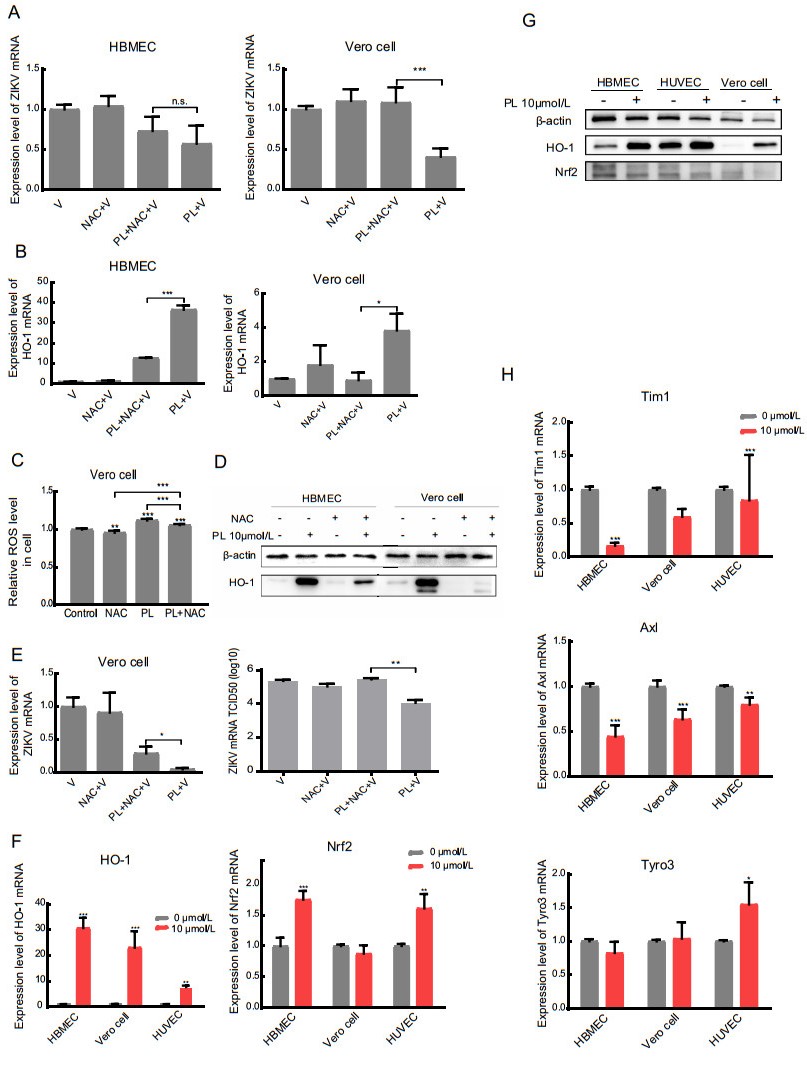
Figure 4. inhibits ZIKV replication by increasing ROS, which induces HO-1. A, B RT-qPCR analysis of Zika virus expression and HO-1 in HBMEC and Vero cells. The significance of differences between groups was determined using two-tailed Student's t-tests. C The detection of ROS level in Vero cell. The concentration of NAC was 5 mmol/L. D Western blot analysis of HO-1 protein expression in HBMEC and Vero cell using anti-HO-1. Antibody against b-actin served as the protein loading control. E RT-qPCR analysis and TCID50 of Zika virus in the supernatant of Vero cell treated with NAC (200 nmol/L) 1 h before the treatment of PL. F, H RT-qPCR analysis of HO-1, Nrf2, Tyro3, Tim1 and Axl in HBMEC, HUVEC, and Vero cell in the absence or presence of 10 μmol/L PL 24 h before detection. The significance of differences between groups was determined using one-way ANOVA. G Western blot analysis of HO-1 and Nrf2 in HBMEC, HUVEC, and Vero cell in the absence or presence of 10 μmol/L PL 24 h before detection. b-actin served as the protein loading control. All variables were repeated for at least three times. * P < 0.05 ** P < 0.001 *** P < 0.0001.
The supernatant from Vero cells was tested for viral activity by the TCID50 assay (Fig. 4E). The results showed that virus content in the PL + NAC + Zika virus group was higher than that in the PL + Zika virus group (P < 0.05). Overall, these results indicated that in Vero cells PL-induced HO-1 expression by increasing intracellular ROS, thereby inhibiting the replication of Zika virus.
Nrf2 is one of several important nuclear factors that activate the transcription of HO-1 (Huang et al. 2017). However, in Vero cells treated with PL, there was no significant change in the expression of Nrf2. In contrast, compared to that in the 0 μmol/L group, the relative expression of Nrf2 mRNA in the 10 μmol/L PL group was 1.75 times in HBMECs and 1.61 times in HUVECs (P < 0.05) (Fig. 4F). Interestingly, the difference was far smaller than the changes in HO-1. Specifically, the relative expression of HO-1 in the 10 μmol/L PL group was 30.7, 23.0, and 7.69 (P < 0.05) times that in the 0 μmol/L group in HBMECs, Vero cells, and HUVECs, respectively. This result suggested that the expression of Nrf2 may be unaffected, or slightly affected by PL (Fig. 4F, 4G), indicating that the antiviral effect of PL was not mediated through the Nrf2-HO-1 pathway.
We also measured the relative mRNA expression levels of the Zika virus cofactors Axl, Tim1, and Tyro3 via qPCR (Hamel et al. 2015). As shown in Fig. 4H, the changes in mRNA of these cofactors were minimal (< 2 times) following treatment of the three cell lines with 0 or 10 μmol/L PL for 24 h. This indicated that the inhibitory effect of PL on the replication of Zika virus was not likely achieved by interfering with cofactors involved in viral entry into host cells; however, this conclusion requires further verification.
PL Reduces Zika Virus RNA Replication and Upregulates HO-1 Expression in a Concentration- and Time-dependent Manner
The PL-Induced Increase in HO-1 Expression Inhibits Replication of Zika Virus
PL Increases HO-1 Expression by Increasing ROS and Inhibiting Replication of Zika Virus mRNA
-
There are many studies on the pathogenesis of Zika virus, but there is still no effective treatment for Zika virus infection. Therefore, it is critical for the prevention of an epidemic to develop effective drugs and vaccines that can inhibit Zika virus. In this study, we analyzed the expression of Zika virus, and HO-1 in three types of Zika-infected cells (HBMECs, Vero cells, and HUVECs) treated with PL. We confirmed that PL inhibited the replication of Zika virus, a finding that can be useful for future drug development. The inhibitor we used in this study (PL) induced the expression of an endogenous host protection factor, HO-1. Previous studies have shown that PL inhibits the proliferation of various tumor cells, such as glioma, gastric cancer, and lung adenocarcinoma cells, mainly by increasing intracellular ROS, which are highly bioactive and involved in multiple biological effects (Bezerra et al. 2007; Raj et al. 2011; Duan et al. 2016). Importantly, PL is not toxic to primary cells, and studies have shown that it exerts neuroprotective effects by inhibiting neuroinflammatory reactions (Gu et al. 2017).
Both HBMECs and HUVECs are endothelial cells. Such cells have a barrier function where they inhibit harmful components in the blood from entering neurons, retinas, testes, placentas, and other compartments (Greenwood et al. 2011; Tang et al. 2015; Goasdoué et al. 2017). The blood–brain barrier, formed by brain capillaries, consists of unique brain microvascular endothelial cells that limit the migration of immune cells and viruses to neurons (Bergelson 2009; Eigenmann et al. 2013). Studies have shown that Zika virus can infect and reproduce in HBMECs, thereby crossing the blood–brain barrier, causing inflammatory reactions, and damaging brain tissue (Goasdoué et al. 2017). In addition, studies have found that Zika virus can infect human dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells.
DC-SIGN, Axl, Tyro3, and TIM1 promote the entry of Zika virus into cells. Among these factors, Axl plays a major role (Hamel et al. 2015). Studies of primary human neural and placental cells have shown a correlation between Axl expression and Zika virus infection (Nowakowski et al. 2016; Tabata et al. 2016). However, another report proposed that Axl was unlikely to act as a receptor for the entry of Zika virus, but may instead promote the infection of Zika virus in human astrocytes by antagonizing type I interferon signaling (Chen et al. 2018a). In this study, we measured the expression of Axl, Tim1, and Tryo3 in HBMECs, Vero cells, and HUVECs after treatment with 0 or 10 μmol/L PL. We found that, as PL concentration increased, there were only minor differences in the expression levels of these three receptors. Moreover, from the relative expression of Zika virus at 0 h shown in Fig. 1C–1E, it can be observed that there was no change in the amount of virus adsorbed by the cells. Thus, the inhibitory effect of PL on Zika virus was not mediated at the stage of host cell entry, but rather at the stage of virus replication. Related studies have reported that, in the treatment of newly discovered Zika virus-susceptible target cells (i.e., primary culture of human monocyte-derived macrophages) with heme to induce HO-1 expression, Zika virus replication was inhibited.
Some studies suggested that Nrf2 activates innate host defenses by inducing HO-1, thereby inhibiting Zika virus replication (Huang et al. 2017). However, our current results revealed that, after PL treatment, there was no significant difference in the relative expression of Nrf2, suggesting that its expression may not be affected by PL. Thus, the antiviral effect of PL was not mediated by the Nrf2-HO-1 pathway.
There are also reports that question the role of HO-1 in inhibiting Zika virus replication. The researchers mentioned that, when Zika virus replicated in A549 and HEK- 293A cells, HO-1 expression was significantly downregulated, thereby inhibiting its antiviral effect (El Kalamouni et al. 2018). However, based on the results of our study, after 12 h of infection, when the amount of Zika virus had already reached a high level, the mRNA and protein expression of HO-1 was not inhibited. We also infected Vero cells with ZIKV at an MOI of 0.1, 1 and 10, which showed the similar results that the level of HO-1 mRNA was not inhibited. This difference may have occurred because we used different cell lines. Both A549 and HEK- 293A cells are epithelial cells, whereas HBMECs and HUVECs are endothelial cells. Furthermore, though Vero cells are epithelial cells, they are also Zika virus-susceptible cells.
HBMECs and HUVECs are important components of the human blood–brain and placental barriers, and these two barriers are closely related to the neuropathogenicity of Zika virus. In addition, it was reported that the inhibitory effect of HO-1 was prominent when the MOI of Zika virusinfected cells was 5. Although we only used an MOI value of 1, the amount of virus after 24 h of infection was at least 10 times higher than that at 0 h, and there was still no inhibition of HO-1 expression. Moreover, other experimental results agree that HO-1 inhibited the replication of Zika virus, further supporting a key role for this enzyme. To further confirm this, future studies should explore how to inhibit Zika virus replication with HO-1, as HO-1 is not affected by Zika virus. Moreover, our results indicated that there might be other mechanisms that inhibit the replication of Zika virus besides the upregulation of HO-1 expression after treatment with PL. These mechanisms should be further investigated in future studies.
Our results showed that, in HBMECs and Vero cells, the mRNA and protein expression levels of HO-1 were downregulated at 12 h after transfection with HO-1 siRNA, indicating that the siRNA was effective. In Vero cells, when the expression of HO-1 was downregulated, the replication of Zika virus increased to some extent. In HBMECs, however, this effect was not evident. The main reason for this may be that HBMECs have a low basal expression level of HO-1 (i.e., at the mRNA level, the Ct value of qPCR was approximately 30; at the protein level, western blots only showed a weak band). In this case, even if HO-1 expression was further reduced, its effect on viral replication would be small.
When we pretreated cells with NAC, the ROS scavenger, the HO-1 level in cell was significantly reduced. But after treated with PL, HO-1 was not completely cleared by NAC. So, the ZIKV mRNA level of PL + NAC + ZIKV group was lower than NAC + ZIKV group. In general, the intracellular ROS was increased by treating with PL and induced HO-1 expression.
In summary, PL induced oxidative stress in cells by increasing ROS. This, in turn, induced an increase in the expression of HO-1, thereby inhibiting Zika virus. However, other factors may also be involved, and thus the precise mechanism underlying the action of PL is still not fully understood. Although further research is required, these findings provided new clues for drug research on the prevention and treatment of Zika virus.
-
We are grateful for Changwen Ke of the Disease Control and Prevention, Guangdong, China, for Zika virus Z16006. This work was supported by the National Natural Science Foundation (Nos. 31670168, 31470271 and 81730110), National Key R&D Program of China (Grant No. 2018YFC1602206) and Guangdong Provincial Science and Technology (No. 2018B020207006).
-
BZ and WZ conceptualized the study. WL, LS, and JG did formal analysis. WL and JG did investigation. BZ provided methodology. HZ, YH, and JC provided visualization. WL and JG drafted the manuscript. BZ, WZ, CW, and XW supervised the study. All authors read and approved the final version of the manuscript.
-
The authors declare no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
















 DownLoad:
DownLoad: