HTML
-
The host innate immune system inhibits viral infection by producing interferon (IFN). Pathogen-related molecular pattern (PAMP) is recognized by cellular pattern recognition receptor (PRR) to promote IFN expression and secretion. Many interferon-stimulated genes (ISGs) play a role by inhibiting specific stages of the virus life cycle (Liu et al. 2013). Cholesterol-25-hydroxylase (CH25H) is a membrane protein associated with endoplasmic reticulum, and it is an interferon-stimulated factor regulated by interferon (Lv et al. 2019). Previous studies have shown that the expression of CH25H is increased after macrophages are infected with murine cytomegalovirus (MCMV), and that the CH25H mRNA is up-regulated in macrophages and dendritic cells after being induced by IFN, suggesting that CH25H is induced by IFN, and it plays a role in inhibiting virus replication (Park and Scott 2010; Shibata et al. 2013). In addition, CH25H is a multitransmembrane endoplasmic reticulum (ER)-related enzyme which catalyzes excess cholesterol to produce 25-hydroxycholesterol (25HC), further reducing cholesterol accumulation. The resultant 25HC can inhibit sterol biosynthesis by regulating the activities of nuclear receptor and sterol response element binding protein (SREBP) (Kandutsch et al. 1978; Janowski et al. 1999).
Recent studies have found that metabolism and innate immunity are interrelated. Viruses can promote their own proliferation by changing cell lipid metabolism, and virus replication, maturation, and secretion are related to the pathways inhibiting cholesterol and fatty acid biosynthesis (Bordier et al. 2003; del Real et al. 2004; Munger et al. 2008; Blanc et al. 2011; Clark et al. 2012). The 25HC widely suppresses enveloped viruses such as vesicular stomatitis virus (VSV), herpes virus (HSV), West Nile virus (WNV), human immunodeficiency virus (HIV), murine gammaherpesvirus (MHV68) and acutely pathogenic Ebola virus (EBOV), Rift Valley fever virus (RVFV), Russian spring-summer encephalitis virus (RSSEV), Nipah viruses, and Zika virus (Liu et al. 2013; Li et al. 2017). The 25HC also has antiviral activity against non-enveloped viruses contain poliovirus and human papillomavirus-16 (HPV-16) (Civra et al. 2014). In addition to its antiviral activity, when macrophages were treated with 25HC, the expressions of IL-lb and inflammatory corpuscle were decreased, and the expressions of IL-6 and IL-8 were increased (Koarai et al. 2012; Gold et al. 2014; Reboldi et al. 2014).
Seneca Valley virus (SVV) is a positive single-stranded RNA virus belonging to the Picornaviridae family (Borghese and Michiels 2011). Its nucleotide sequence is highly similar to those of Cardiovirus genus members (Hales et al. 2008; Venkataraman et al. 2008). SVV is originally isolated from cultured cells as a contaminant. The relationship between porcine SVV infection and porcine idiopathic vesicular disease (PIVD) was reported for the first time in 2007. The infected pigs showed symptoms such as claudication, blistering, depression, anorexia, and fever, which could not be distinguished from the clinical symptoms caused by foot and mouth disease virus (FMDV), swine vesicular disease virus (SVDV), vesicular stomatitis virus (VSV), and vesicular exanthema of swine virus (VESV) (Sáiz et al. 2003; Fernández et al. 2008; Lung et al. 2011; Leme et al. 2015; Bracht et al. 2016). SVV can infect pigs at different ages, and sows and growing pigs have a very low mortality rate, but the mortality rate of piglets is as high as 30% ~ 70% (Leme et al. 2016). Exploration of the interaction between virus and host in SVV will help to develop more effective control measures, such as the development of effective vaccines and antiviral drugs. As a new pathogen of pigs, SVV's interaction with CH25H has not been reported yet.
In the present study, we investigated the effect of CH25H gene on SVV replication by overexpressing or knocking down mouse CH25H (mCH25H). The results indicated that overexpression of mCH25H significantly inhibited SVV replication. Further, knockdown of CH25H enhanced SVV replication. Our data revealed that the mCH25H mutants (mCH25H-M) lacking hydroxylase activity lost the ability to suppress SVV replication, suggesting that mCH25H depended on enzyme activity to exert an antiviral function. The results of 25HC experiment in vitro confirmed that CH25H suppressed SVV infection by producing 25HC to block adsorption process.
-
Baby hamster kidney (BHK-21) cells and human embryonic kidney (HEK-293T) cells were grown at 37 ℃ in Dulbecco's modified essential medium (DMEM, Invitrogen, Carlsbad, CA, United States) supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen, Grand Island, NY, United States). SVV HB-CH-2016 (SVV-HB) strain was proliferated and preserved in our laboratory (Qian et al. 2016, 2017). UV-SVV-HB was obtained by fully irradiating the SVV-HB virus under a UV lamp for half an hour.
-
The mCH25H expression plasmids were constructed by inserting mouse CH25H (mCH25H) gene into pTRIP- 3FLAG vector or pCAGGS-HA vector. The human- and swine-derived CH25H eukaryotic expression plasmids were also constructed by inserting hCH25H and sCH25H gene into pCAGGS-HA vector. The mCH25H knockeddown HEK-293T cell lines were established by transfecting mCH25H-targeting shRNA (shCH25H-3#: 50-CAGTTTAACTGCAATTTTGCTCC-30; shCH25H-4#: 50- TACACTTGATCCAGAAGAAGAAA-30) into the cells. The mouse CH25H catalytic mutant (mCH25H-M) was established by converting histidine residues 242 and 243 into glutamines (Lund et al. 1998).
-
Mouse monoclonal antibodies against HA-tag (M180-3) and FLAG-tag (M185-3L) were purchased from Medical and Biological Laboratories CO., LTD. (MBL, Japan). Rabbit anti-SVV VP1 polyclonal antibodies were prepared by our laboratory. The monoclonal antibody (mAb) against CH25H was purchased from Abnova (J4011-1G8). Mouse anti-alpha Tubulin monoclonal antibody (66,031-1-Ig) was purchased from Proteintech Group Inc. (China). Horseradish peroxidase-conjugated (HRP) goat anti-mouse (BA1051) and goat anti-rabbit IgG (BA1054) (H + L) secondary antibodies were obtained from Boster Bioengineering Ltd (China). Alexa Fluor® goat anti-mouse IgG and Alexa Fluor® goat anti-rabbit IgG were obtained from Invitrogen (USA).
-
Total RNA was extracted with the TRIZOL reagent (Invitrogen, Grand Island, NY, USA) according to the manufacturer's instructions. Total RNA (1.0 μg) was reverse transcribed using First Strand cDNA Synthesis Kit (TOYOBO) according to the manufacturer's instructions. The mRNA levels of the target genes were determined through relative quantitative real-time (RT) PCR by using SYBR Green Real-time PCR Master Mix (TOYOBO), and fluorescent signals were analyzed by an ABI StepOne Plus system (Applied Biosystems). All reactions were performed in triplicate, and the mRNA level of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous reference control.
-
HEK-293T cells were transfected with the indicated plasmids. At 24 h post transfection, the cells were harvested and lyzed in lysis buffer containing 50 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% (v/v) NP40, and protease inhibitors (Roche, United Kingdom). The samples were centrifuged for 10 min at 4 ℃ to remove cellular debris. The protein concentrations of whole-cell lysates were measured using a bicinchoninic acid protein assay kit (Thermo Scientific). Equal amounts of proteins were electrophoresed on 12% sodium dodecyl sulfate polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Roche, United Kingdom). The membranes were blocked with 5% (v/v) non-fat milk dissolved in Trisbuffered saline containing 5% (v/v) Tween-20 (DGBio, Beijing, China) for 4 h at room temperature (RT). The membranes were subsequently incubated with diluted primary antibodies at RT or at 4 ℃ for 2 h or 16 h, respectively. HRP-conjugated anti-rabbit or anti-mouse IgGs were used as secondary antibodies. An enhanced chemiluminescent substrate was used for membrane detection (Thermo Scientific, United States). All immunoblot images were obtained using a Bio-Rad ChemiDoc XRS+ instrument and imageJ software. Expression of alpha Tubulin was determined using anti-alpha Tubulin monoclonal antibodies as internal references.
-
Plaque assay was performed with BHK-21 cell lines growing to 60%-80% (6 × 105 cells/well) in 6-well culture plates (Nunc). The 800 μL ten-fold serial dilution of virus was incubated with PBS-washed cells at 37 ℃ for 90 min. After virus removal, the cells were rewashed and replenished with 2% LMP agrose (Low Melting-point agrose) dissolved in DMEM containing 2% FBS and 1% Penicillin streptomycin solution (Genview) to 2 mL. After 2 days, the cells were fixed with 10% (v/v) formaldehyde, subsequently stained with 0.2% (m/v) crystal violet for 3 h. The plaque-forming units (PFU) were counted manually for virus-infectivity titer analysis after ddH2O rinsing. Data from three independent experiments were expressed as mean ± SD (standard deviation).
-
MCE (Med Chem Express, US) CCK-8 (Cell Counting kit-8) was used for cytotoxicity assay. HEK-293T cells were transfected with pCAGGS-HA-mCH25H or pCAGGS-HAvector at indicated doses for 24 h, and the diluted medicine 25HC was incubated with 1 × 104 BHK-21 cell lines in 96-well plates. After incubation for 72 h, 10 μL of CCK-8 reagent was added into each well. One hour later, the absorbance at 450 nm was measured after being gently mixed in a shaker for 1 min to ensure homogeneous distribution of color.
-
SVV-HB was incubated with 25HC (5 μmol/L and 10 μmol/L) or DMSO at 37 ℃ for 1 h, and then the cell media were replaced with the resultant mixture of SVV-HB (MOI of 1) and 25HC or DMSO. After incubation for 1.5 h at 37 ℃, cells were washed and covered with low-temperature agar, and mixed (1:1) with 2 × DMEM, and then incubated at 37 ℃ for another 48 h. The virus titer was determined by counting the number of plaques.
-
BHK-21 cells cultured in 12-well plates were pre-chilled at 4 ℃ for 1 h, and then the media were replaced with a mixture of 25HC (5 μmol/L and 10 μmol/L) or DMSO and SVV-HB (at an MOI of 5). After incubation at 4 ℃ for another 2 h, cells were washed and harvested for plaque assay and qRT-PCR assay.
-
An unpaired, two-tailed Student's t-test was performed to reveal statistically significant difference between treatment groups and the control group at three levels of *P < 0.05, **P < 0.01, and ***P < 0.001. The experiments were repeated three times.
Cells and Viruses
Plasmids
Antibodies
Quantitative RT-PCR
Western Blot Assay
Plaque Assay
Cytotoxicity Assay
Inactivation Assay
Adsorption Assay
Statistical Analysis
-
To explore the effect of SVV-HB infection on CH25H, HEK-293T and BHK-21 cells were infected with SVV-HB at an MOI of 1 or treated with DMEM, and then incubated for indicated hours. The results of Western blot analysis and qRT-PCR assay indicated that the expressions of CH25H protein and mRNA were decreased after SVV-HB infection (Fig. 1). Together, our data illustrated that CH25H expression was downregulated by SVV-HB infection.
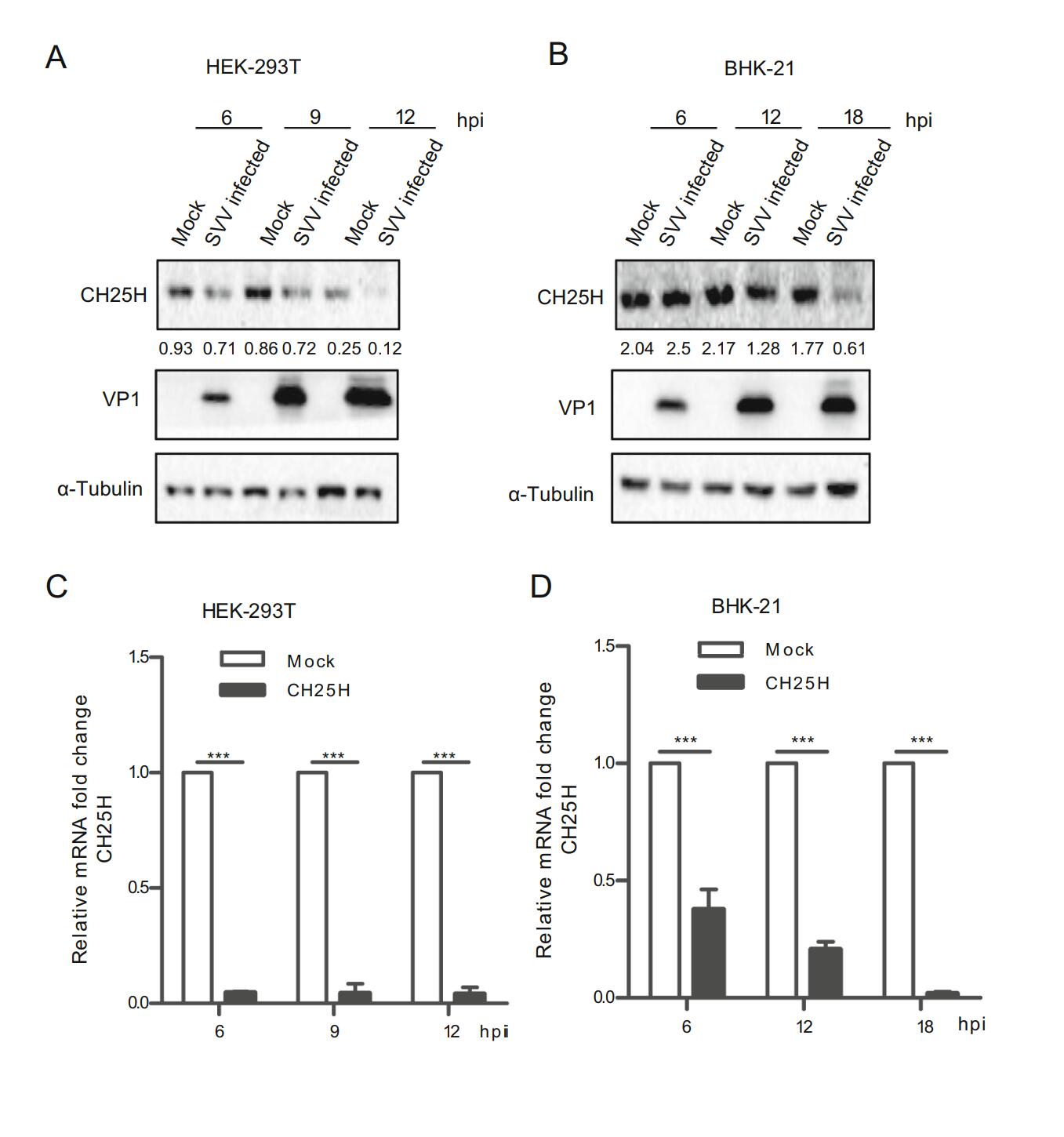
Figure 1. CH25H is downregulated upon SVV-HB infection. HEK- 293T and BHK-21 cells were infected with SVV-HB (MOI of 1) for indicated hours. The cells were harvested to analyze CH25H protein expression by Western blot with anti-CH25H monoclonal antibody (A) and anti-VP1 polyclonal antibody (B) and CH25H mRNA level was detect in HEK-293T cells (C) and BHK-21 cells (D) by qRT-PCR assay. GAPDH is used as the internal control. All the results were confirmed by three independent experiments. ***P < 0.001.
-
The cellular viability was measured using a CCK-8 Kit, the results showed that different doses of CH25H have no effect on HEK-293T cell viability (Fig. 2A). HEK-293T cells were transfected with the indicated doses of mCH25H or vector alone, respectively, followed by the infection with SVV-HB at an MOI of 1. Afterwards, cells and supernatants were harvested, respectively at 3, 6, and 9 h after infection. SVV-HB protein (VP1) was examined by Western blot assay (Fig. 2B). The plaque assay showed that overexpressed mCH25H cells exhibited significantly decreased virus titers, compared with control cells (Fig. 2C). SVV VP1 mRNA was analyzed by quantitative reverse transcription-PCR (qRT-PCR) analysis (Fig. 2D). As expected, overexpression of mCH25H could significantly suppress SVV-HB replication at all indicated time points.
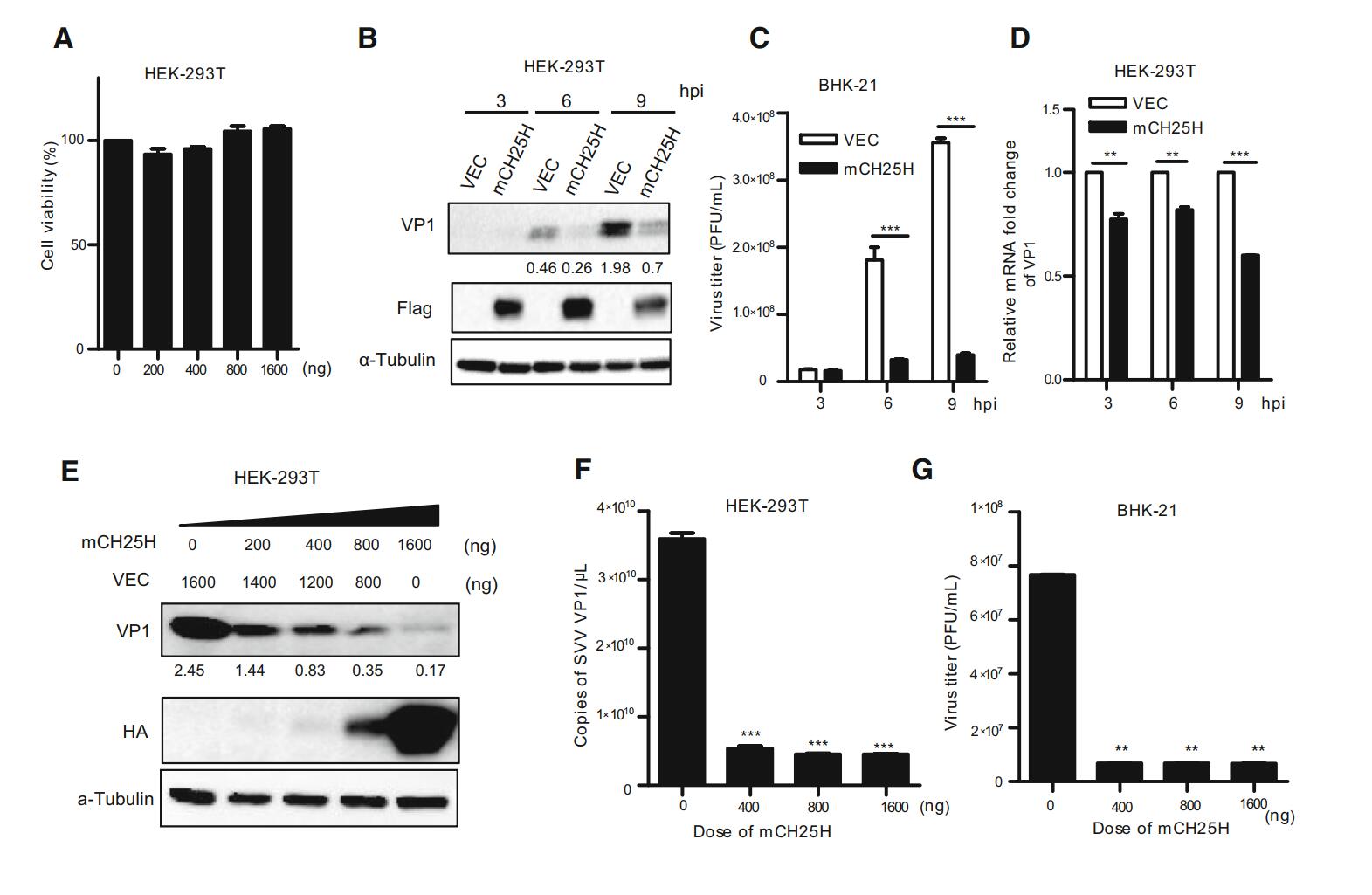
Figure 2. Overexpression of mCH25H suppresses SVV-HB replication. A HEK-293T cells were transfected with mCH25H expression plasmid or empty vector at indicated doses for 24 h, the cellular viability was measured using a CCK-8 Kit. B HEK-293T cells were transfected with mCH25H expression plasmid or empty vector for 24 h, following infection with 1 MOI of SVV-HB and VP1 was detected at 3, 6, and 9 h post infection. The supernatants were harvested to analyze virus titers by plaque assay (C), and cells were harvested to analyze by qRT-PCR assay (D). HEK-293T cells were transfected with mCH25H expression plasmid or empty vector at the different doses, then infected with SVV-HB (MOI of 1) for 9 h and cells were fixed to detect by Western blot assay (E) and qRT-PCR assay (F), and the supernatants were harvested to analyze virus titers by plaque assay (G). The results represent the means and standard deviations from three independent experiments. **P < 0.01, and ***P < 0.001.
Further, whether the ectopic expression of mCH25H has effect on SVV-HB replication in a dose-dependent manner was investigated. HEK-293T cells were transfected with different doses of pTRIP-3FLAG-mCH25H plasmids and the negative control, respectively, followed by infection with SVV-HB (MOI of 1) for 9 h. The results indicated that SVV-HB replication was inhibited in a dose-dependent manner after transfection with mCH25H (Fig. 2E-2G).
-
To further explore the antiviral potential of endogenous CH25H, the mCH25H knockdown cell line was established by transfecting mCH25H targeting shRNA into HEK-293T cell line. CH25H expression exhibited different degrees of decrease after transfection with four shRNAs, with shCH25H-3# and shCH25H-4# displaying better knockdown effect on protein level (Fig. 3A). qRT-PCR assay and Western blot analysis indicated that cells transfected with shCH25H-3# and shCH25H-4# displayed lower levels of CH25H and higher levels of SVV-HB at 6 and 9 h after infection than negative control (Fig. 3B and 3C).
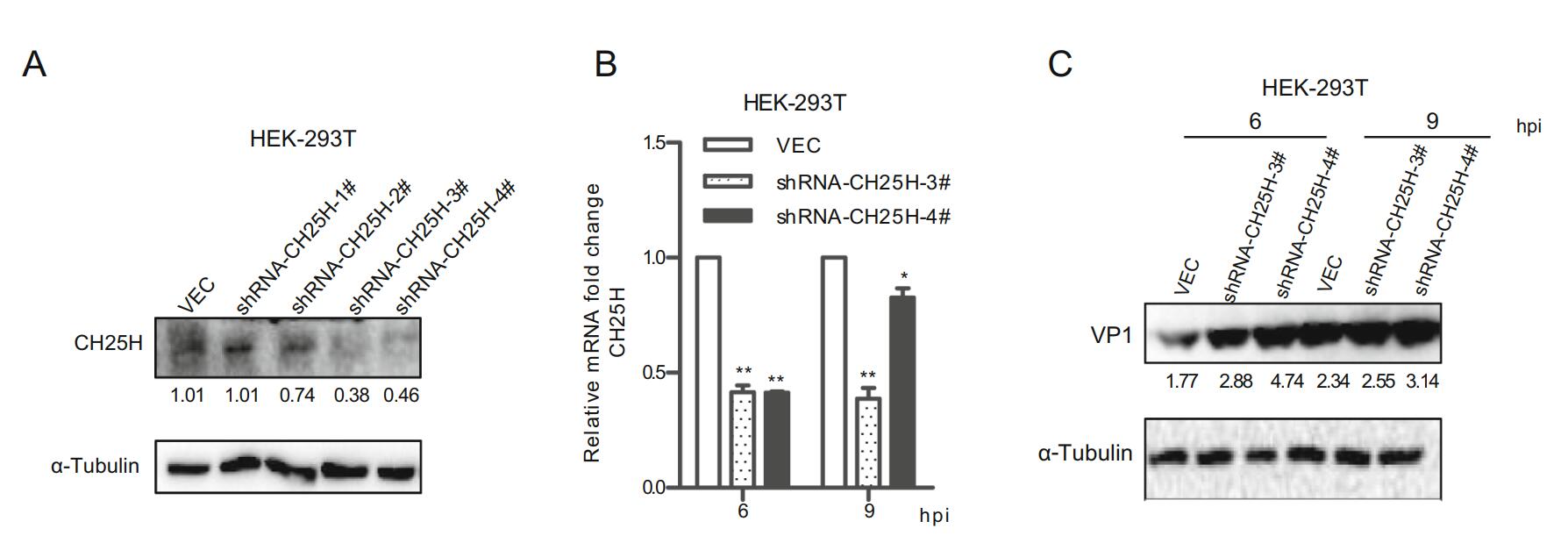
Figure 3. Knockdown of CH25H enhances SVV-HB replication. A HEK-293T cells were transfected with CH25H-specific shRNA or control vector for 24 h, cells were harvested to analyze by Western blot assay. HEK-293T cells were transfected with shRNA-CH25H-3#, shRNA-CH25H-4# or empty vector for 24 h, cells were infected with SVV-HB (MOI of 1). And then the cells were harvested to analyze by qRT-PCR assay (B) and Western blot assay (C). *P < 0.05, **P < 0.01.
-
In order to explore whether the inhibition of SVV replication by CH25H was species-specific, HEK-293T cells were transfected with different species of CH25H or vector, respectively (Fig. 4). At 24 h post transfection, the supernatants were replaced with DMEM solution containing 2% FBS and SVV-HB (MOI of 1). Cells and supernatants were harvested at the indicated time points. The results indicated that SVV-HB replication was inhibited at protein and mRNA level in human-, mouse-, swinederived groups than in negative control group (Fig. 4A and 4B). The plaque assay also showed that the virus titers were lower in CH25H overexpression groups than in negative control group (Fig. 4C). These data confirmed that there is no species difference in the inhibition of SVV-HB replication by CH25H.
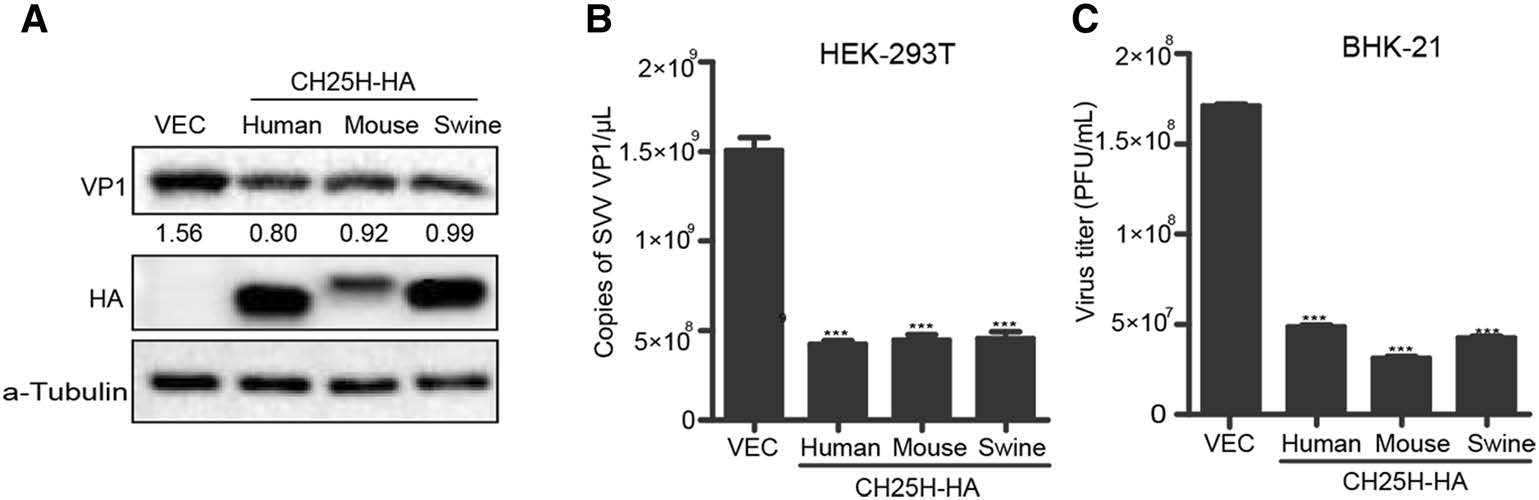
Figure 4. CH25H from different species inhibits SVV-HB replication. HEK-293T cells were transfected with human, mouse or swine CH25H expression plasmids or empty vector for 24 h, the cells were infected with SVV-HB (MOI of 1) for 9 h. And cells were harvested to analyze by Western blot assay (A) and qRT-PCR assay (B), then the supernatants were harvested to analyze virus titers by plaque assay (C). The results represent the means and standard deviations from three independent experiments. ***P < 0.001.
-
To explore whether the enzyme activity of CH25H was indispensable for its own antiviral activity against SVV-HB, a mouse CH25H catalytic mutant (mCH25H-M) was generated by converting histidine residues 242 and 243 to glutamine (Lund et al., 1998). HEK-293T cells were transfected with pTRIP-3FLAG plasmid, pTRIP-3FLAGmCH25H plasmid, or mCH25H-M for 24 h, and then infected with SVV-HB at an MOI of 1. After incubation for another 9 h, the cells were collected and analyzed by Western blot assay. As shown in Fig. 5A, the protein level of SVV VP1 transfected with mCH25H-M was similar to that transfected with vector pTRIP-3FLAG, while that transfected with mCH25H was found to be reduced. Subsequently, after HEK-293T cells were transfected with mCH25H-M or vector for 24 h, and then infected with SVV-HB at an MOI of 1, the supernatants and cells were collected at different time points. The Western blot assay and plaque assay results showed that overexpression of mCH25H-M had no effect on SVV-HB replication (Fig. 5B and 5C). We also explored the effect of different doses of mCH25H-M on SVV replication. The results indicated that mCH25H-M lost the ability to inhibit SVV-HB replication (Fig. 5D and 5E).
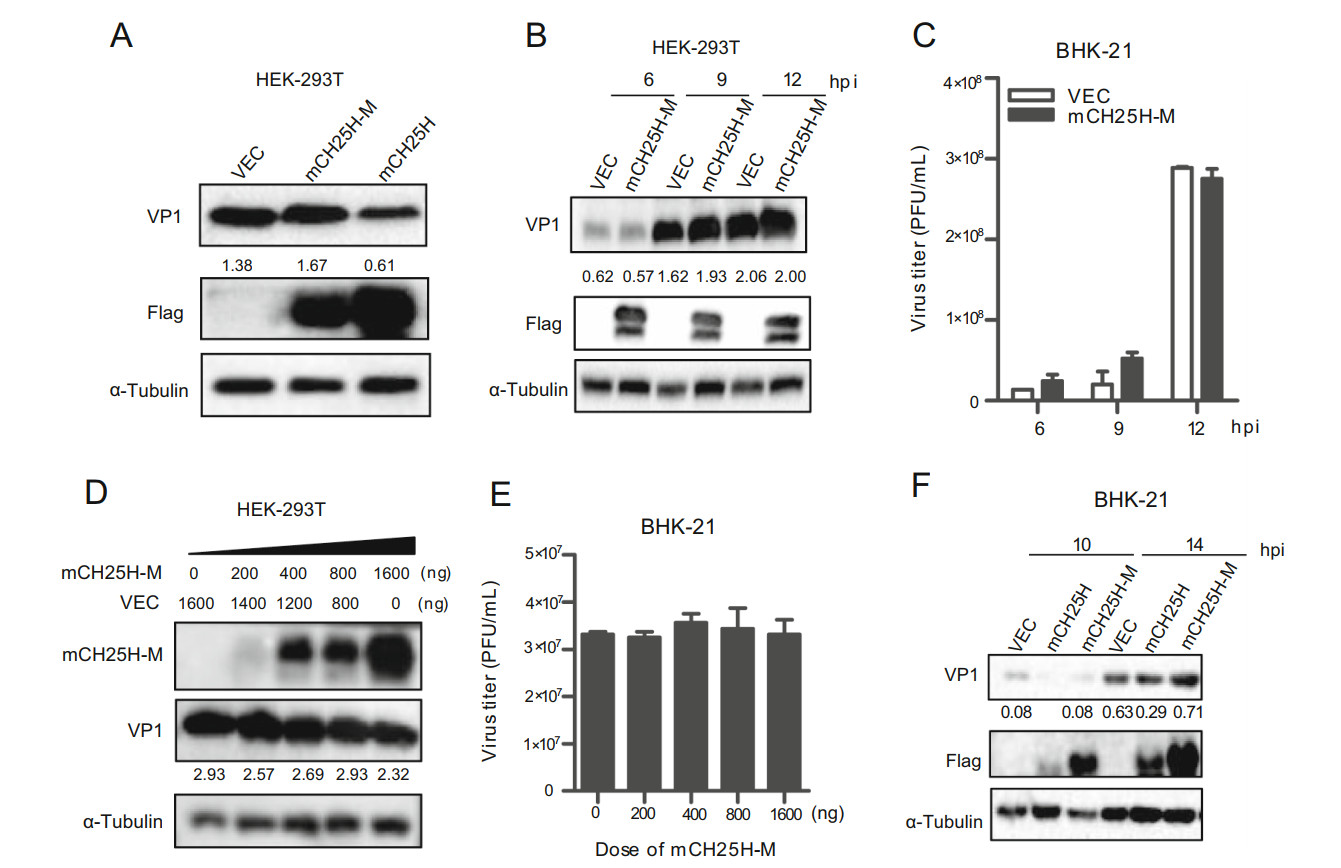
Figure 5. CH25H mutant loses ability to inhibit SVV-HB replication. HEK-293T cells were transfected with the mCH25H, mCH25H-M expression plasmid or empty vector, and then infected with SVV-HB (MOI of 1) for 9 h (A) for VP1 detection by Western blot assay, or the cells were harvested at 6, 9, and 12 hpi (B) and the supernatants were harvested to analyze virus titers by plaque assay (C). D HEK-293T cells were transfected with mCH25H-M expression plasmid at indicated doses following infection with SVV-HB (MOI of 1) for 9 h, cells were harvested to analyze by Western blot assay. The supernatants were harvested to analyze virus titers by plaque assay (E). F BHK-21 cells were transfected with the mCH25H, mCH25HM expression constructs or empty vector following infection with SVV-HB (MOI of 1), cells were harvested to analysis by Western blot assay. All the results were confirmed by three independent experiments. Error bars represent the standard deviations of triplicate experiments.
We further investigated whether overexpression of mCH25H-M and mCH25H could inhibit SVV-HB replication in BHK-21 cells, and we observed similar results that mCH25H rather than mCH25H-M inhibited SVV-HB (Fig. 5F). Taken together, our data showed that mCH25H-M was deprived of its ability to suppress SVV-HB replication.
-
Previous studies reported that CH25H exerted antiviral activity by producing 25HC (Wu et al. 2018). To further investigate whether the antiviral function of CH25H was dependent on its enzyme activity, 25HC was directly added into the culture medium. Then, cell cytotoxicity assay was conducted to evaluate the cytotoxicity of synthesized 25HC towards BHK-21 cells. The cell viability assay results showed that cytotoxicity could not be detected until 15 μmol/L 25HC were used in BHK-21 cell lines (Fig. 6A). BHK-21 cells were pretreated with 5 μmol/L 25HC or dimethyl sulfoxide (DMSO, as the negative control) for 12 h, and then infected with SVV-HB at an MOI of 1. At indicated hours (10, 14, and 18 hpi), the cells were harvested for Western blot analysis. The results showed that 25HC inhibited SVV-HB replication at all time points (Fig. 6B). We also tested the anti-SVV-HB efficiency of different doses of 25HC (1.25 μmol/L and 5 μmol/L). The plaque assay and qRT-PCR assay showed that 25HC inhibited SVV-HB replication in a dose-dependent manner (Fig. 6C and 6D). Together, these data demonstrated that the catalytic product of CH25H, 25HC, can restrict SVV infection in BHK-21 cells.
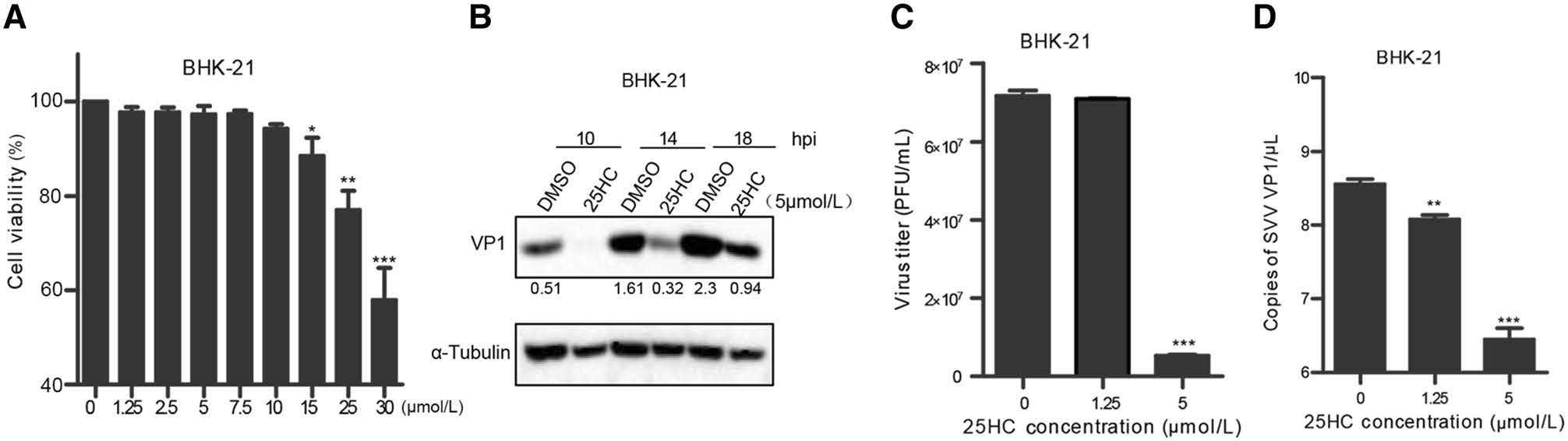
Figure 6. 25HC inhibits SVV replication. A BHK-21 cells were pretreated with 25HC at the indicated concentrations (1.25, 2.5, 5, 7.5, 10, 15, 25, and 30 μmol/L) for 48 h, the cellular cytotoxicity of 25HC was measured using a CCK-8 Kit. BHK-21 cells were pretreated with 25HC or DMSO at 5 μmol/L for 12 h, then infected with SVV-HB (MOI of 1) for indicated hours. The cells were harvested to analyze by Western blot assay (B), supernatants were harvested to analyze virus titers by plaque assay (C). VP1 mRNA of SVV-HB was detected by qRT-PCR assay (D). All the results were confirmed by three independent experiments. Error bars represent the standard deviations of triplicate experiments. *P < 0.05, **P < 0.01, and ***P < 0.001.
-
Further assay was conducted to identify the process when 25HC exerted its antiviral effect in the virus life cycle. The virus inactivation assay results indicated that 25HC failed to kill SVV-HB virions directly (Fig. 7A). Next, we explored at which stage of the SVV replication cycle 25HC exerts its effect. BHK-21 cells were pre-chilled at 4 ℃ for 1 h, then 25HC or DMSO was added and followed by SVV mixture incubation at 4 ℃ for another 2 h, the adsorption assay results demonstrated that 25HC blocked viral adsorption (Fig. 7B and 7C). The penetration assay was performed in BHK-21 cells, and the results showed that 25HC treatment prior to SVV-HB infection did not block virus penetration (Fig. 7D). The BHK-21 cells were infected with SVV-HB at an MOI of 1 for 12 h, and then the virus-containing medium was replaced with fresh 25HC (5 μmol/L and 10 μmol/L) or DMSO medium. Subsequently, BHK-21 cells were incubated for 2 h or 6 h, washed and covered with fresh media for 1 h, and then the cells and supernatants were harvested for Western blot assay and virus titer assay. Our data suggested that 25HC had no effect on SVV-HB replication or release (Fig. 7E and 7F). Taken together, 25HC reduced SVV-HB infection by blocking SVV-HB virion adsorption, but it had no effect on the penetration, replication, and release steps during the viral replication cycle.

Figure 7. 25HC blocks SVV adsorption. A The SVV-HB, UV-SVV-HB, and SVV-HB treated with different concentrations of DMSO or 25HC were detected by plaque assay. BHK-21 cells cultured in 12-well plates were prechilled at 4 ℃ for 1 h, and then the media were replaced by a mixture of 25HC (5 μmol/L and 10 μmol/L) or DMSO and 1 MOI of SVV-HB. After incubated at 4 ℃ for another 2 h, the cells were washed and harvested to analyze virus titers by plaque assay (B) and qRT-PCR assay (C). D BHK-21 cells cultured in 12-well plates were prechilled at 4 ℃ for 1 h and then incubated for another 2 h at 4 ℃ with 1 MOI of SVV-HB. The virus-containing medium was replaced by fresh 25HC or DMSO medium for 2 h at 37 ℃, the cells were harvested for qRT-PCR assay. E BHK-21 cells were infected with 1 MOI of SVV-HB for 12 h, the cells were collected to analyze by western blot assay and harvested the supernatants to analyze virus titers by plaque assay (F) after treatment with different concentrations of DMSO or 25HC for 2 h or 6 h. **P < 0.01, and ***P < 0.001.
Expression of CH25H is Downregulated after SVV-HB Infection
mCH25H Suppresses SVV-HB Replication
Knockdown of CH25H Enhances SVV-HB Replication
CH25H from Different Species Inhibits SVV-HB Replication
CH25H Mutant Loses Ability to Inhibit SVV-HB Replication
25HC Inhibits SVV-HB Replication
25HC Blocks SVV-HB Adsorption
-
Innate immunity is the first line of defense against virus infection, and it plays a key role in the host immune response to virus clearance. Type Ⅰ IFN and its induced cellular antiviral response are the main defense mechanisms against virus infection in the early stage. As an ISG, CH25H plays an important regulatory role in host lipid metabolism and antiviral immune response (Raniga and Liang 2018). For example, the stimulation of type Ⅰ and Ⅱ IFN could induce the early expression of CH25H in macrophages and could significantly inhibit the proliferation of enveloped viruses such as HIV, VSV, and EBOV by affecting the fusion of virus and host cell membrane (Liu et al. 2013). This study results showed that CH25H was downregulated after HEK-293T and BHK-21 cell infection with SVV-HB, however, the mechanism by which SVV degrades CH25H protein remains to be further explored. To assess the effect of CH25H on SVV replication, we analyzed the anti-SVV mechanism of CH25H via overexpression and knockdown of this gene in HEK-293T cells and BHK-21 cells. The results confirmed that overexpression of mCH25H could inhibit SVV replication, while knockdown of CH25H enhanced SVV replication.
Previous studies have reported that CH25H affects the proliferation of different viruses through different mechanisms in 25HC-dependent or 25HC-independent manner. For example, as a member of oxysterols, 25HC may directly modify the cell membrane to block the entry of VSV. The change in cholesterol content in the cells has been reported to block the lipid valve to reduce the entry of PRV virions (Desplanques et al. 2008). The 25HC inhibits Lassa virus infection through aberrant GP1 glycosylation (Shrivastava-Ranjan et al. 2016). The previous study of CH25H and HCV indicated that CH25H mutant could inhibit HCV replication by degrading NS5A dimer, whereas CH25H could block host lipid metabolism by producing 25HC, thus affecting the transcriptional activity of sterol regulatory element binding protein (SREBP), further acting on the late stage of HCV replication cycle, eventually effectively inhibiting virus infection (Chen et al. 2014). Herein, we demonstrated that the mCH25H mutants lost the ability to inhibit SVV replication, but 25HC strongly inhibited SVV replication in BHK-21 cells in vitro, suggesting that CH25H resists SVV infection in a 25HC-dependent manner. This study found that 25HC exerted its antiviral function during virus adsorption process. Considering the relationship between cell metabolism and innate immunity, other oxygenated steroids might also have antiviral activity in vitro. 27HC inhibited the infection of viruses such as mouse cytomegalovirus, human papillomavirus, human rotavirus, and human rhinovirus. Interestingly, the expression of 27-hydroxylase did not respond to interferon stimulation (Civra et al. 2014). The 24HC could block mouse cytomegalovirus infection, and 22 (S) HC could inhibit hepatitis B virus replication. As a non-enveloped virus, anti-SVV 25HC does not involve membrane fusion, which may be due to the fact that 25HC affects the stability of lipid valves or the biosynthesis of sterols. The effects of other oxysteroids and cholesterol metabolism on SVV replication remain to be further studied.
In summary, this study reveals for the first time that CH25H suppresses SVV infection in a 25HC-dependent manner. Since 25HC is a natural product of lipid metabolism and can prevent virus entry by modifying the host membrane. Our results confirm that 25HC inhibits SVV infection by blocking virus adsorption process, therefore it has great potential to be a first-line anti-SVV drug.
-
This work was supported by the National Natural Science Foundation of China (31772749, 31572495), the Fundamental Research Funds for the Central Universities (2662017PY108), and Natural Science Foundation of Hubei Province (2019CFA010). Great gratitude goes to linguistics Professor Ping Liu from Foreign Language College, Huazhong Agriculture University, Wuhan, China for her work at English editing and language polishing.
-
PQ, HC, and XL designed the research integrated the data, proofread and revised the manuscript. HL, ZZ, and LQ designed and performed the research, collected and analysed the data. HL drafted the manuscript. PQ finalized the manuscript. All authors read and approved the final version of the manuscript to be published.
-
The authors declare no conflict of interest.
-
This article does not contain any study with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: