HTML
-
Human adenoviruses (HAdVs) belong to the genus Mastadenovirus in the family Adenoviridae, which have a double-stranded DNA genome of 33–36 kb. It is a highly contagious pathogen that mainly infects human respiratory tract, digestive tract, and urogenital tract, and is widely distributed in nature. Adenoviruses can cause acute respiratory disease (ARD), epidemic keratoconjunctivitis (red eye disease), acute pharyngeal conjunctivitis, infantile and fatal bronchial pneumonia, gastroenteritis, cystitis and other diseases (Cao et al. 2014; Lion 2014; Pasarica et al.2008; Zhang et al. 2006; Zhao et al. 2014). The infections in immunocompetent individuals are usually mild and selflimiting, but more and more severe and even fatal cases in children and immunocompetent adults caused by emergent and re-emergent HAdV types have been reported (Cao et al. 2014; Cui et al. 2015; Girouard et al. 2013; Hage et al. 2014; Jones et al. 2007; Zhao et al. 2014).
To date, more than 100 genotypes of HAdVs have been identified and classified into 7 species: species A to G (Ji et al. 2021; Jones et al. 2007). Of the seven HAdV species, species B (mostly HAdV-B3, -B7, -B14, and -B55), species C (HAdV-C1, -C2, -C5) and species E (HAdV-E4) are the major HAdVs associated with ARD in children and adults and have caused lots of outbreaks of pneumonia and pharyngoconjunctivitis in China (Walsh et al. 2010; Zhang et al. 2019, 2012a, 2006; Zhao et al. 2014).
HAdV-B55 is a newly identified re-emergent ARD pathogen (Walsh et al. 2010; Zhang et al. 2012a). Complete genome analysis suggested that this new type, HAdV-B55, is a Trojan horse, which contains a recombinant genome from both a renal pathogen, HAdV-B11, which accounts for 2.6%, and a respiratory pathogen, HAdV-B14, which accounts for 97.4% (Walsh et al. 2010; Zhang et al.2016a). HAdV-B55 can cause respiratory diseases in the clinic, showing the tissue tropism of respiratory viruses, but the neutralizing antibodies produced are directed against kidney adenovirus type 11 (Cheng et al. 2018). The seven hypervariable regions (HVR, 907 bp) of hexon proteins are derived from HAdV-B11, which can stimulate the body to produce specific neutralizing antibodies, and the rest of the genome is all from HAdV-B14, which confers the cell tropism, biological and pathogenicity properties (Chmielewicz et al. 2005; Yang et al. 2009). The evolutionary rates of hexon, fiber, and whole genome of HAdV- 55 were estimated at 6.2 9 10–5 s/s/y, 8.0 9 10–5 s/s/y, and 1.7 9 10–5 s/s/y, respectively (Lu et al. 2014).
HAdV-B55 was first identified as an atypical HAdV-B11 strain in Spain in 1969 (Hierholzer et al. 1974). It then appeared in a Turkish military camp in 2004, where hundreds of military trainees fell ill and one man died (Chmielewicz et al. 2005). In recent years, this ARD pathogen has reemerged several times, once in Singapore in 2005 (Kajon et al. 2010), and in China, HAdV-B55 first broke out and caused 264 infections in Shanxi Province in 2006 and was misidentified as type 11a (Yang et al. 2009). Since the emergence of the first HAdV-B55 strain QSDLL, there have been more than 10 HAdV-B55-associated ARD outbreaks in China cities, such as in Shanxi, Chongqing, Beijing, Jiangsu, Hebei, Jinan, Tianjin, and Guangdong, etc. (Cao et al. 2014; Chen et al. 2016; Gao et al. 2018; Gu et al. 2012; Li et al. 2014; Sun et al. 2014; Tan et al. 2016; Walsh et al. 2010; Yang et al. 2009; Yi et al. 2017; Zhang et al. 2012a), with severe and some fatal cases (Cao et al. 2014; Jing et al. 2019; Sun et al. 2014; Tan et al. 2016; Zhang et al. 2016b; Zhu et al. 2009). In northern China, the study showed that HAdV-B55 had higher pneumonia severity index scores in adolescent and adult patients compared with those with other types (Cao et al. 2014). Similar ARD outbreaks were also reported in other countries (Geng et al. 2013b; Lafolie et al. 2016; Salama et al. 2016). HAdV-B55 also caused household transmission of severe adult community-acquired pneumonia (CAP) with fatality (Jing et al. 2019). HAdV-B55 outbreak in a neurosurgical inpatient department of a hospital of Guangdong Province, China was also reported (Yi et al. 2017). In military recruits and high school students, HAdV-B55 was also identified (Chmielewicz et al.2005; Geng et al. 2013a; Zhu et al. 2009). More and more ARD outbreaks and fatal cases associated with HAdV-B55 infections have aroused increasing public health concern about HAdV variants (Yu et al. 2016).
The disease severity might be associated with the virus tropisms. It is likely that differences in fiber capsid proteins that mediate cell receptor binding and cell entry contribute to serotype-specific differences in clinical manifestations. Fiber of HAdVs determines which receptor the virus uses, and the interaction with the cellular receptor controls whether the virus can enter the infected cell. Adenovirus fiber protein is an oligomeric molecule comprised of three identical polypeptide subunits of 30–65 kDa (Signas et al.1985). The fiber contains a conserved N-terminal tail that mediates interaction with penton base, a variable-length elongated domain (shaft), and a C-terminal knob that mediates high-affinity interaction with cell receptors (Philipson et al. 1968). The three-dimensional structure of the Ad2 and Ad5 fiber N-terminal knob domain has been determined. Originally shown as the receptor for adenoviruses 2 and 5 (Jeffrey 1997), Coxsackie-adenovirus receptors (CAR) is a member of the immunoglobulin superfamily expressed on multiple cell types (Roelvink et al. 1998). It is a receptor for a diverse range of adenoviruses across the species, such as species A, C, D, and F.
However, not all HAdVs use CAR to bind host cells. Desmoglein 2 (DSG2), a calcium-binding transmembrane glycoprotein that is overexpressed in a series of tumor epithelial cells. In epithelial cells of the respiratory tract, gastrointestinal tract and urethra, DSG2 is a component of the cell–cell adhesion structure (Chitaev and Troyanovsky 1997). It is well known that in addition to the function of maintaining cell adhesion, DSG2 is also involved in intracellular signaling (Harmon and Green 2013). Its cytoplasmic tail interacts with a range of proteins that are in direct contact with cell adhesion and intercellular junction/cell morphology modulators (Cowin 1994). It has been reported to interact at high affinity with human adenoviruses type 3 and 14 (Vassal-Stermann et al. 2018; Wang et al. 2015, 2011).
CD46, also called MCP (Membrane Cofactor Protein), is a complement regulatory protein expressed on the surface of almost all nucleated cells (Andrews et al. 1985). The fiber-knob domain of HAdV-B11 had been determined in complex with the crystal structure of the CD46 extracellular domain (Persson et al. 2010). Because CD46 is upregulated in numerous cancers, it remains a popular choice for the development of oncolytic virotherapies (Uhlen et al. 2017). HAdV-B3, -B7, -B11 and -B35 could also bind to CD46 and use CD46 as a receptor (Marttila et al. 2005; Segerman et al. 2003) (Cupelli et al. 2010; Fleischli et al. 2007; Gaggar et al. 2003; Wang et al. 2007).
In addition, there are many other proteins that act as candidate receptors for adenoviruses. For example, CD80 and CD86 are the secondary receptors for HAdV-B3 (Short et al. 2006, 2004); sialic acid is the candidate receptor for HAdV-D37 and HAdV-G52 (Annasara Lenman et al. 2017, 2018); HAdV-D26 uses sialic acids as a primary receptor to enter cells (Baker et al. 2019); Blood Coagulation Factor X (Alba et al. 2009; Kalyuzhniy et al. 2008; Waddington et al. 2008), HSPG (Di Paolo et al. 2007; Kritz et al. 2007), Integrin (Bai et al. 1993; Henning et al.2005), and MARCO (Stichling et al. 2018) are also receptors for HAdV-C5.
However, the receptor of HAdV-B55 pathogen that has caused dozen of ARD outbreaks in China is not clear. It is necessary to confirm whether the receptor of newly discovered HAdV-B55 recombinant between HAdV-B11 and HAdV-B14 has been altered or whether the affinity between this recombinant adenovirus and DSG2 has been changed. This is of great significance for further elucidating the mechanism of reproduction and immunity of HAdV-B55 at the molecular level, understanding of the relationship between adenovirus and host cells, and development of effective adenovirus vaccines and antiviral drugs.
-
HAdV-B3 GZ01 strain (GenBank Accession Number. DQ099432), HAdV-B14 GZ01 strain (GenBank Accession Number. JQ824845.1) and HAdV-B55 BJ01 strain (GenBank Accession Number. JX491639.1) were isolated, identified and preserved in our laboratory (Cheng et al.2018; Zhang et al. 2017, 2012a, 2012b, 2009, 2006). HAdV-B11 (GenBank Accession Number. 011202.1) was purchased by ATCC (ATCCⓇ VR-12TM). The recombinant adenoviruses rAd3EGFP (HAdV-3-GFP) and rAd5△E3GFP (HAdV-5-GFP) was constructed in our laboratory (Zhang et al. 2009, 2006). The recombinant adenovirus rAd55EGFP (HAdV-55-GFP) was kindly provided by Guangzhou Institutes of Biomedicine and Health (Guangzhou, China).
All the adenoviruses except the replication-defective HAdV-C5 (cultured in Ad293 cells) were inoculated into A549 cells, and cultured at 37 ℃ in an atmosphere containing 5% (v/v) carbon dioxide. A549 and Ad293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin (P/S), 2 mmol/L glutamine (Glu). All viruses were purified by two rounds of CsCl density gradient centrifugation, dialyzed against 10 mmol/L Tris–Cl (pH 8.0), 150 mmol/L NaCl and 1 mmol/L MgCl2 and then stored at -80 ℃ in 10% (v/v) glycerol (Stichling et al. 2018).
-
Activation of carbocyanine dye (Cy3 dye): Cy3 fluorochromes (CyDye mono-reactive NHS Esters, Soulebao, Beijing) was dissolved in DMSO at a concentration of 10 mg/mL, then add EDC (1-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydrochloride) and NHS (N-Hydroxy succinimide) directly, make the final ratio: CY3:EDC: NHS = 1:1.2:1.2 (Eq). After mixing, the Cy3 activation system was shaking at room temperature for 1–2 h.
Labeling was performed by conjugating dye to virus at a concentration approximately equal to 1012 virus particles/mL, where reconstituted Cy3 dye was 20% of the final solution (Miyazawa et al. 1999). The mixture was kept at room temperature for 30 min in the dark with gentle mixing every 10 min; then the reaction system was added to the dialysis membrane, and dialysis is carried out overnight at 4 ℃ (Leopold et al. 1998). After dialysis overnight, change the dialysis buffer and continue for at least 3 h; the labeled virus was removed and glycerol was add up to the final concentration of 30%, and stored at -80 ℃. Cy3-conjugated virus preparations were used to infect the cells and imaged by confocal microscopy at indicated times post infection (Zhou et al. 2012).
-
A549 or mouse embryo fibroblast cells (NIH/3T3) were cultured in confocal dishes (Nest; Wuxi), then cells were incubated with Cy3-conjugated HAdV-B55 at 100 pfu/cell for 20 min at 37 ℃, washed twice with ice-cold PBS and then fixed with methanol/aceton (1:1 vol/vol) for 15 min at 4 ℃ or with 4% paraformaldehyde for 30 min at 4 ℃. After fixation, cells were washed with PBS twice and blocked with 500 lL PBS/1% BSA powder for 1 h at room temperature. Anti-desmoglein 2 rabbit polyclonal (abcam: ab96761) was diluted in PBS (1:1000) and incubated with the cells at 4 ℃ overnight. Then, secondary goat antirabbit Alexa-Fluor488-conjugated antibody (Abcam: ab150077) was applied after three times of PBS wash for 45 min at room temperature. After three times of PBS wash, confocal images were taken on Nikon A1? Confocal Microscope using 2009 or 4009 lenses.
-
The total RNA of A549 cells was extracted using RNAiso Reagent Kit (TaKaRa), and reverse transcripted into cDNA with primer DSG-R using SuperScriptTM IV One-Step RTPCR System (Thermo Fisher, Shanghai). Primer DSG-F: 5'-GGAGGATCCGCCACCATGGCGCGGAGCCCG-3', DSG-R: 5'-GACCTCGAGTTAGGAGTAAGAATGCTG TACAGTGCTA-3'. Restriction enzyme sites of BamH1 and Xho1 were added in the 5' of the primers (underlined), respectively. The DSG2 PCR product was cloned in pcDNA3.1(+) by double restriction and ligation. Positive clones were selected to transfection into 3T3 cells at a density of 1×105 per well in a 24-well imaging plate using LipofectamineTM LTX Reagent with PLUSTM Reagent (Thermo Fisher, Shanghai). One day after transfection, 3T3 cells were infected by HAdV-55-GFP, HAdV- 3-GFP and HAdV-5-GFP (0.1 MOI), respectively. Then the green fluorescence was observed at 48 h post infection.
-
A set of DSG2 specific siRNA was synthesized by Sangon Biotech (Shanghai; China). The siRNA sequences were as follow: CAAUAUACCUGUAGUAGAA, GAGAGGAUCUGUCCAAGAA, CCUUAGAGCUACGCAUUAA and CCAGUGUUCUACCUAAAUA (Wang et al. 2011). siRNA transfection was performed using LipofectamineTM LTX Reagent with PLUSTM Reagent (Thermo Fisher, Shanghai).
A total of 1×105 A549 were transfected with 1 μg DSG2 siRNA or control siRNA. Forty eight hours after siRNA transfection, cells were infected with 1 MOI of HAdV-3-GFP, HAdV-5-GFP and HAdV-55-GFP, respectively, then GFP expression was analyzed 18 h later (Wang et al. 2011). For HAdV-11 and -14, indirect immunofluorescent assay was used to analyze the fluorescence signal (Haralambieva et al. 2008). The fluorescence signal intensity in the cells was recorded and calculated using Image J (http://rsb.info.nih.gov/ij/index.html).
-
A549 cell membrane proteins were extracted at 24 h, 48 h and 72 h after siRNA transfection, respectively. The protein extraction was followed by the manufacturer's protocol without modifications (Membrane Protein Extraction Kit, Bestbio, Shanghai).
SDS-PAGE precast gels with 10% polyacrylamide gradient were used. A total of 1 μg of protein mixed with 2 × loading buffer (10 mmol/L Tris–HCl, pH 6.8, 200 mmol/L dithiothreitol, 4% SDS, 20% glycerol, 0.2% bromophenol blue) was loaded per lane after boiled for 10 min. The following running buffer was used: 25 mmol/L Tris, pH 8.3, 0.192 mol/L glycine, and 0.1% SDS. After electrophoresis, proteins were transferred to nitrocellulose membrane and incubated with the corresponding anti-bodies. For detection of DSG2 binding, filters were incubated with DSG2 antibody (Rabbit polyclonal antibody, catalog number: 21880–1-AP, Proteintech) and HRP-conjugated goat anti-rabbit IgG antibody (catalog number: SA00001-2, Proteintech).
-
Swiss model (https://www.swissmodel.expasy.org/interactive) was used to analyze the structure. HAdV-B55 fiber domain were predicted using HAdV-C5 (SMTL ID: 1qiu.2) as a reference sequence. Monomers of HAdV-B55 fiber domain mutant were pointed with black arrows.
-
All results are expressed as means ± standard deviations (SD). One-way analysis of variance (ANOVA) for multiple testing was applied using GraphPad Prism, version 7.00, for Windows (GraphPad Software). The P values are indicated in the figure legends. P values < 0.05 were considered statistically significant.
Viruses and Cell Lines
Labeling of HAdV-B55 by Cy3 Conjugation
Immunofluorescence and Confocal Microscopy
Construction of the Plasmid Expressing DSG2 in 3T3 Cells
siRNA Assay
Western Blot
Three-dimensional (3D) Structure
Statistical Analysis
-
Adenoviruses have strong tissue tropism, and the fiber gene determines which receptor the virus uses. The fiber amino acid sequences of HAdV-B14p de Wit, HAdV-B14p1 GZ01 and HAdV-B55 BJ01 were aligned. Compared to HAdV-B14p prototype strain de Wit, the fiber gene of HAdV-B14p1GZ01 strain has a deletion of two consecutive amino acids (lysine and glutamic acid) in the knob region, which is a typical characteristic of HAdV-B14p1 genotype (Wang et al. 2009). HAdV-B14p1 uses the same receptor DSG2 as HAdV-B14p prototype strain de Wit (Gustafsson et al. 2006; Wang et al. 2009). Compared to the prototype HAdV-B14p de Wit, the fiber of HAdV-B55 has two amino acid mutations (A84T and E116K) in the Shaft region and one amino acid mutation (V138I) in the knob region (Fig. 1A). These three mutation may change the receptor attachment of HAdV-B55 as they are located in the exposed surface of fiber protein (Fig. 1B) as predicted by Swiss Model using HAdV-C5 (SMTL ID: 1qiu.2) as a reference sequence.
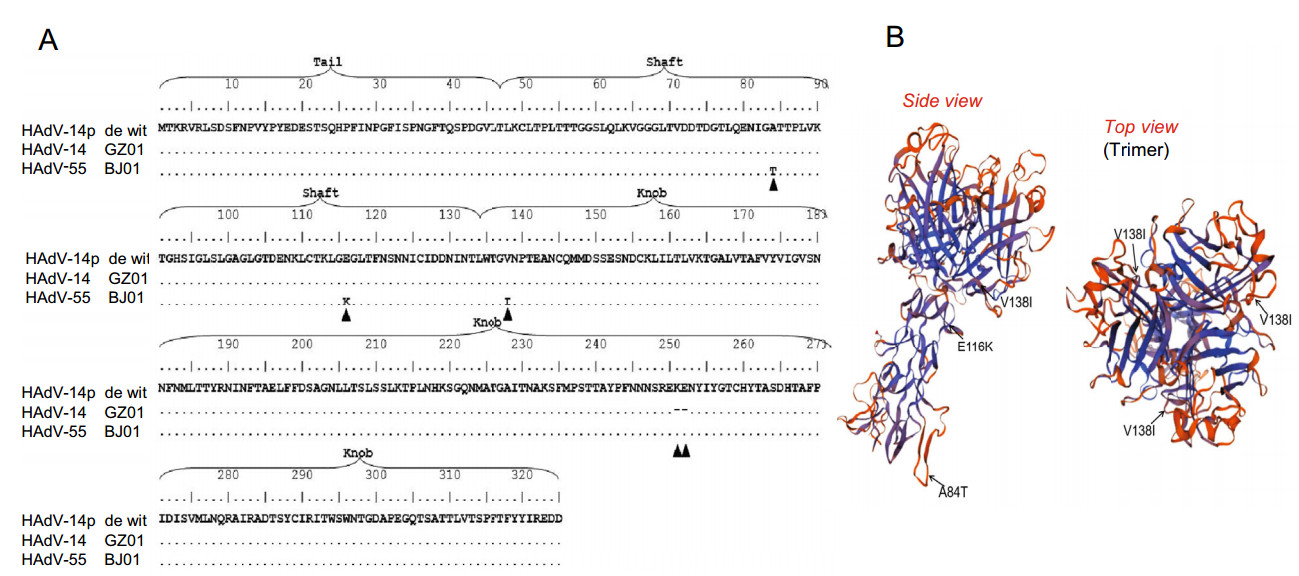
Figure 1. The alignment and structural analysis of HAdV-B55 fiber protein. The alignment of HAdV-55 fiber gene (A) and the homology model of HAdV-B55 fiber protein (B). A The same amino acid sequence after the alignment is represented by ''.'', Mutations and deletions are represented by ▲. The three mutations are A84T, E116K and V138I. B The structure of fiber is analyzed by Swiss model (https://www.swissmodel.expasy.org/interactive). HAdV-B55 fiber domain were predicted using HAdV-C5 (SMTL ID: 1qiu.2) as a reference sequence. Monomers of HAdV-B55 fiber domain mutant were pointed with black arrows. A84T is in the tall domain, E116K is in the shaft domain and V138I is in the knob domain, which are all exposed on the surface of fiber.
-
Wang et al. had shown previously that HAdV-B3 could enter A549 cells because of a specific amino acid in the fiber knob, the primary ligand for host cell binding (Wang et al. 2011). Therefore, we compared cellular entry of Cy3- conjugated HAdV-B55 by confocal immunofluorescence microscopy studies, with 3T3 cells as a negative control. The 3T3 cells transfected with pcDNA3.1-DSG2 were also tested 48 h post transfection. Twenty minutes post infection of A549 cells with Cy3-conjugated HAdV-B55, as expected, both DSG2 in green and Cy3-conjugated HAdVB55 in red were found co-localized in cell membranes (Fig. 2A), while at the same time no green DSG2 and red Cy3-conjugated HAdV-B55 were seen in 3T3 cells infected by HAdV-B55, suggesting that 3T3 cells had no DSG2 in the membranes and were insusceptible to HAdV-B55 infection. However, in the 3T3 cells transfected with pcDNA3.1-DSG2, the green fluorescence was observed in the cytoplasm, indicating that DSG2 protein was expressed in 3T3 cells. Cy3-conjugated HAdV-B55 in red was found to interact with the membrane of 3T3 cells (the position indicated by the white arrows). Tomography showed threedimensional information, and the animation was generated (Supplementary video). In stacked XZ and YZ image sections (Fig. 2B), DSG2 appeared at the distal end of intercellular junctions, and the orange bands which showed the interaction between DSG2 and HAdV-B55 could be observed in A549 cells.
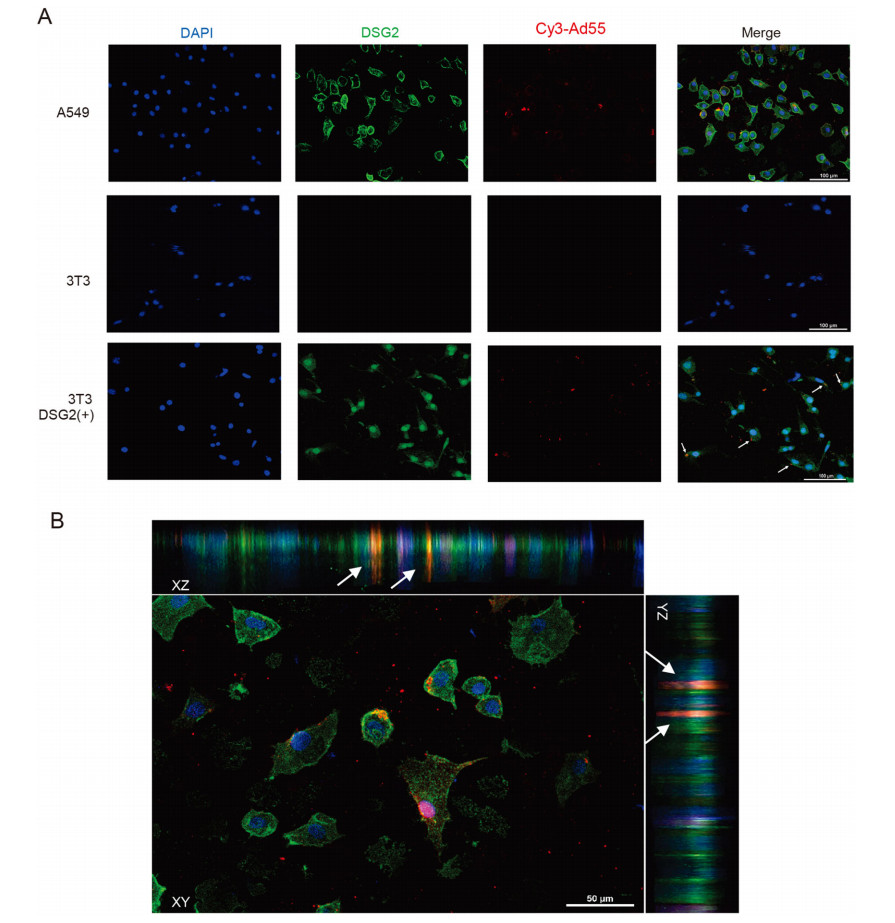
Figure 2. HAdV-B55 can infect A549, and also 3T3 cells expressing DSG2, but not wildtype 3T3 cells. A Comparison of infection by Cy3- conjugated HAdV-B55 in A549 cells, 3T3 cells without DSG2 expression, and 3T3 cells transfected with pcDNA3.1-DSG2 by confocal microscopy at 20 min post infection. DAPI stains nuclei blue, DSG2 green and Cy3-conjugated virus red. Cy3-conjugated HAdV-B55 were indicated by white arrows. Scale bar = 100 μm; B XY, XZ and YZ planes in A549 cells infected by HAdV-B55 are shown respectively. Cy3- conjugated HAdV-B55 were indicated by white arrows.
-
Mouse embryo fibroblast cells (3T3) were transfected with the recombinant plasmid pcDNA3.1-DSG2 expressing the human DSG2 protein. 24 h post transfection, they were infected with HAdV-3-GFP and HAdV-55-GFP (5 FFU/cell), respectively. 3T3 cells infected by HAdV-5- GFP, and A549 cells infected by HAdV-3-GFP and HAdV- 55-GFP were as the positive control. Correspondingly, 3T3 cells directly infected by HAdV-3-GFP and HAdV-55-GFP were as the negative control. 48 h post infection of HAdV- 3-GFP or HAdV-55-GFP, green fluorescence was found in 3T3 cells transfected with pcDNA3.1-DSG2 (Fig. 3A and 3B). HAdV-5-GFP can infect normal 3T3 cells via the CAR receptor (Fig. 3C), while HAdV-3-GFP and HAdV- 55-GFP cannot infect 3T3 directly (Fig. 3A and 3B), i.e., no green fluorescence was found.

Figure 3. The A549 and 3T3 cells infected by HAdV-55-GFP, HAdV-3- GFP and HAdV-5-GFP viruses, with or without pcDNA3.1-DSG2 transfection. A A549 and 3T3 cells at 48 h post infection by HAdV- 55-GFP without or with pcDNA3.1-DSG2 transfection, B A549 and 3T3 cells at 48 h post infection by HAdV-3-GFP without or with pcDNA3.1-DSG2 transfection. C 3T3 cells at 48 h post infection by HAdV-5-GFP without pcDNA3.1-DSG2 transfection. Scale bar = 100μm.
-
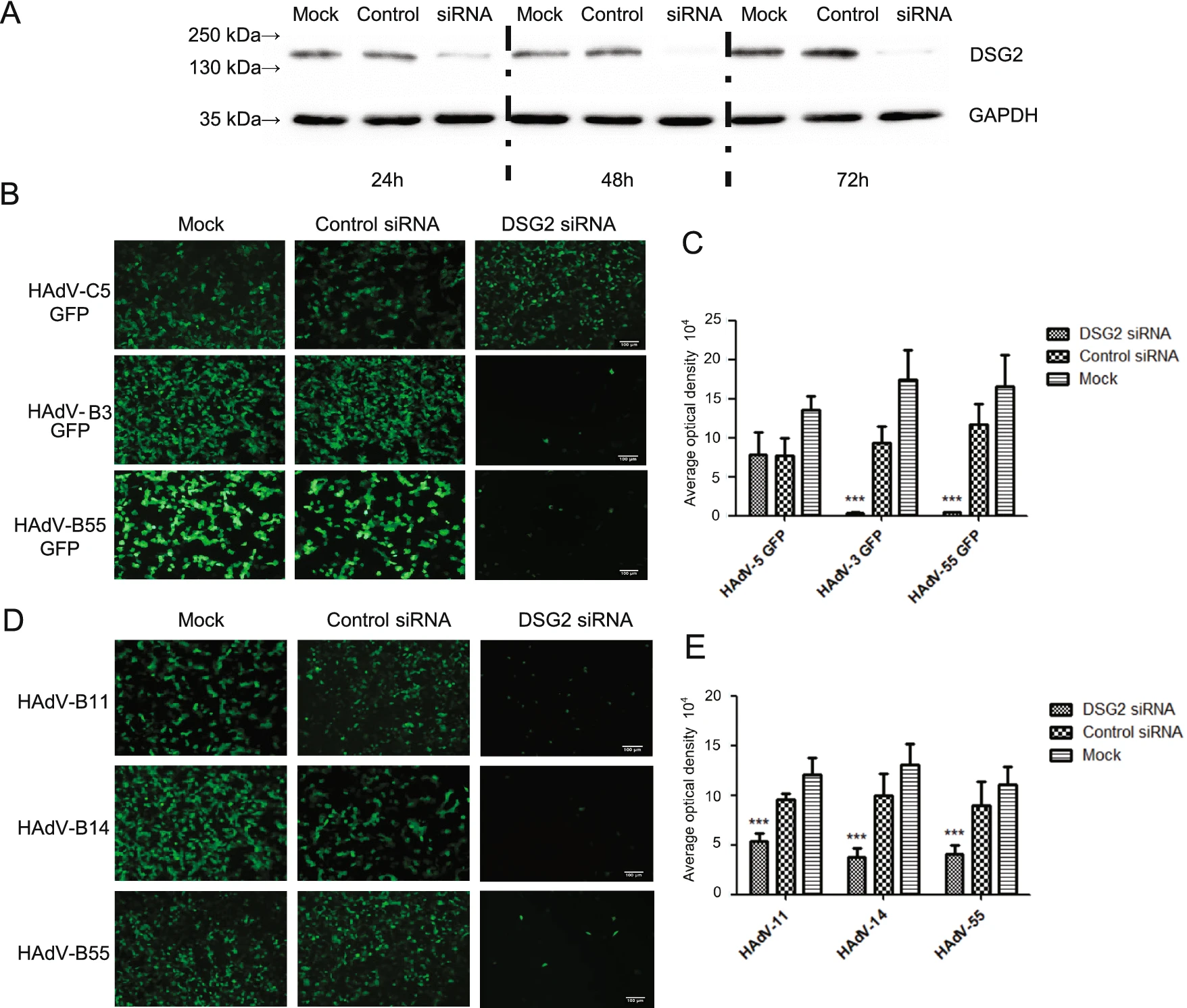
The membrane protein of A549 cells was prepared at 24 h, 48 h and 72 h after transfected with siRNA. Western blot analysis using DSG2-specific antibodies showed that the band is the lightest in 48 h, thus the expression of DSG2 protein reached its lowest level at this time point (Fig. 4A).
The experiment is divided into two parts because of the lack of HAdV-B11/-B14 with GFP. One of which was that A549 cells inhibited by DSG2 siRNA were infected with HAdV-55-GFP or HAdV-3/5-GFP, then the results showed that the fluorescence of the experimental group was significantly decreased (P ≤ 0.01) (Fig. 4B and 4C). The other part was the indirect immunofluorescence, after transfection with DSG2 siRNA, A549 cells were infected with HAdV-B55 or HAdV-B11/B14, then cultured with mouse polyclonal antibody, and secondary anti-mouse Alexa-Fluor 488-conjugated antibody. The results showed that the total fluorescence density of the three viruses was significantly lower than the control group and the negative group (P ≤ 0.01) after DSG2 siRNA knockdown (Fig. 4D and 4E).
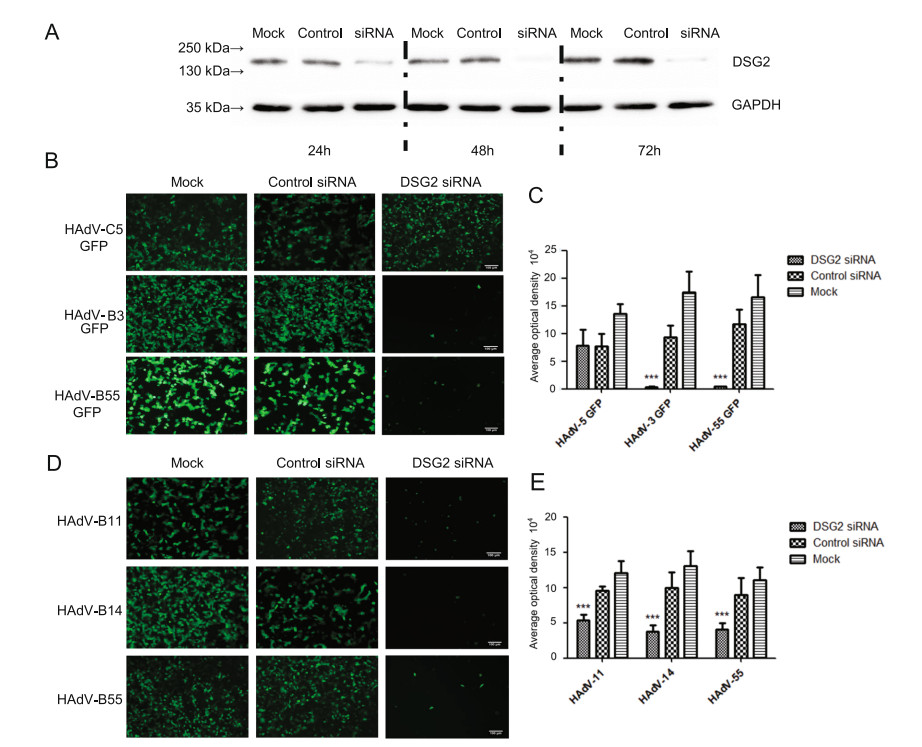
Figure 4. Effect of siRNA-mediated DSG2 downregulation on HAdV attachment. A The Western blot results of DSG2 expression in A549 cells at 24, 48, 72 h after transfection with siRNA. B GFP expression in A549 cells transfected with siRNA and infected with HAdV-55- GFP and HAdV-3/-5-GFP. D The immunofluorescence in A549 cells after infection with HAdV-B55 and HAdV-B11/-B14. Mouse polyclonal antibodies against HAdVs (prepared in our laboratory) were incubated with the cells, then the second antibodies goat Anti-Mouse IgG H & L (FITC) (ab6785) was applied. C and E Mean fluorescence intensity values. n = 10. Note that at 18 h post-infection, GFP and immunofluorescence levels were comparable for HAdV-55-GFP and HAdV-3/-5/-11/-14-GFP. DSG2-siRNA and Control siRNA transfected cells were infected with HAdVs at 1 MOI. ***: P < 0.001. Scale bar = 100 μm.
-
Previous studies have found that the mutation of a key amino acid in fiber can weaken the interaction between the adenovirus and cells and even change the receptor used by the virus (Gustafsson et al. 2006; Huang et al. 1999). For example, a single amino residue in an expose loop of the HAdV-D37 fiber knob could promote virus interaction with its cellular receptor (Huang et al. 1999). Compared to the prototype strain of HAdV-B14 (HAdV-14p de Wit), HAdV-B55 exhibited higher levels of replication in respiratory cells than did either of its parents (HAdV-B14 and -B11) (Liu et al. 2014). The fiber of HAdV-B55 has two amino acid mutations in the shaft region (Ala 84 Thr and Glu 116 Lys) and an amino acid mutation in the knob region (Tyr 138 Ile). These three mutation sites are all on the exposed surface of fiber and can directly interact with viral receptors. Therefore, it is necessary to verify whether the receptor of HAdV-B55 is altered by these three amino acid mutations.
In this study, we found that DSG2 is a major receptor crucial for HAdV-B55 infection. Firstly, we used confocal microscope to visually observe the direct binding of virions to DSG2 protein on the cell membrane. On XZ and YZ axes, orange bands were observed within intercellular junctions (Fig. 2). The mouse embryo fibroblast cells 3T3, nonpermissive rodent cells which do not express DSG2, were able to be infected by HAdV-B55 and green fluorescence was observed when the cells expressed DSG2 after the transfection with pcDNA3.1-DSG2 (Fig. 2 and Fig. 3). As a control, GFP expression was observed in normal 3T3 cells after infection of HAdV-5-GFP, which indicated that HAdV-C5 utilized another receptor not DSG2 to enter 3T3 cells. This clearly widens our understanding of human adenovirus attachment mechanisms. Another finding is that A549 cells with DSG2 knock-down were hard to be infected by HAdV-B3/-B14/-B55, while the cells were still susceptible to HAdV-C5 infection. This further confirmed that the receptor of HAdV-C5 was not DSG2.
Another research team just reported that DSG2 is the primary mediator of HAdV-B55 (Feng et al. 2020). In their loss-of-function studies, they used soluble receptors, blocking antibodies, RNA interference, and gene knockout to demonstrate that DSG2 predominantly mediated HAdVB55 infection. In our study, we performed an additional assay of immunofluorescence confocal microscopy to show three-dimensional binding model of HAdV-B55, and the animation was generated by tomography and showed the interaction between HAdV-B55 and DSG2 (Supplementary video). Our result confirmed that DSG2 is one of the main receptors of HAdV-B55.
In summary, we conclude that the differences in the fiber knob of the HAdV-B14 and HAdV-B55 do not alter the cell tropism of both types of HAdVs. DSG2 is a receptor of HAdV-B55. The better understanding of interactions between adenoviruses and host cells is crucial for the development of RNAi-based new drugs that can interfere with these processes as well as for the development of potent prophylactic vaccines.
The Alignment and Structural Analysis of HAdVB55 Fiber Protein
Binding of HAdV-B55 to DSG2 in A549 Cells and 3T3 Cells Expressing DSG2
HAdV-B3 and HAdV-B55 can Infect 3T3 Cells Expressing Human DSG2
Transfection of DSG2 Specific siRNA Inhibits DSG2 Expression and HAdV-B55 Infection of A549 Cells
Discussion
-
This work was supported by grants from the National Key Research and Development Program of China (2018YFE0204503) and Natural Science Foundation of Guangdong Province (2021A1515010788 and 2018B030312010) as well as from the Guangzhou Healthcare Collaborative Innovation Major Project (201803040004 and 201803040007).
-
QZ designed the experiments. JZ, KM, XW, YJ, SZ, JO, WL, WG, and XW carried out the experiments. JZ, HZ, BY, CW, WZ, JW, and QZ analyzed the data. JZ, JW, and QZ wrote the paper and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors have declared that they have no conflict of interests.
-
This article does not contain any studies with human and animal subjects performed by any of the authors.
















 DownLoad:
DownLoad: