HTML
-
Japanese encephalitis (JE) is an acute inflammatory disease of the central nervous system caused by the Japanese encephalitis virus (JEV), a mosquito-borne flavivirus and a leading cause of epidemic encephalitis in Asia. JEV belongs to the genus Flavivirus of the Flaviviridae family, which includes West Nile virus (WNV), dengue virus (DENV), Zika virus (ZIKV), yellow fever virus (YFN) and tick-borne encephalitis virus (TBEV) (Barrows et al. 2018). As a neurotropic virus, JEV can effectively cross the blood-brain barrier (BBB) to induce acute viral encephalitis syndromes such as fever, coma, seizures, paralysis and even death in humans. While JEV causes high morbidity and mortality, there is no specific and effective therapy available for JE (Erlanger et al. 2009).
Since viruses are obligate intracellular parasites, it is no wonder that they rely on host cell machinery to complete their entire life cycle. Different viruses may adopt different strategies in rewiring host cellular metabolism (Thaker et al. 2019b). However, anabolic phenotype conferred by virus infection is usually common and often similar to tumor cells, like up-regulated nutrients consumption, increased nucleotide and lipid biosynthesis in coping with the high viral needs for proliferative infection (Su et al. 2014; Warburg 1956). Changes take place in cell metabolic state further affect several cell physiological functions, such as cell division and apoptosis. Meanwhile, viruses also have developed multiple strategies to either stimulate or inhibit the metabolism to achieve its desired outcome. However, diverse viruses evolved distinct tactics to cope with different host cells and then lead to dissimilar metabolic landscapes (Mayer et al. 2019). Currently, whether or how JEV interacts with host cells in metabolic facet during infection is still unknown.
As is well-known, JEV is a typical neurotropic virus. Neurons are the dominant cells in brain favored by JEV infection and propagation. Here, we employed untargeted liquid chromatography-tandem mass spectrometry (LCMS)-based metabolomics and Illumina sequencing platform-based transcriptomics to detect metabolites and genes in Neuro2a cells infected with JEV at 24 h post of infection (hpi). A large number of differential metabolites and genes were identified in JEV-infected Neuro2a cells compared to the mock-infected group. Increased glycolysis and its branched pentose phosphate pathway (PPP) flux and impaired oxidative phosphorylation (OXPHOS) in glucose utilization, accompanied with the catabolic pattern of lipid metabolism, were generated to facilitate the biosynthesis of precursors needed for viral replication. Pharmacological inhibitions of both glucose and lipid metabolism could effectively inhibit JEV replication either in Neuro2a cell line or mouse primary neurons. Our results indicated that JEV infection drastically reshaped the metabolic features in neurons to satisfy its own replication. Thus, metabolic facet may be a potential antiviral target in JEV infection.
-
The mouse neuroblastoma cell line Neuro2a and baby hamster kidney (BHK-21) cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Corning, NY, USA) containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (Hyclone, Logan, UT, USA). All cell lines were deposited and thawed in our laboratory. Primary mouse neurons were purchased from JingKang Bio (Shanghai, China). The propagation of JEV wild-type strain P3 in C6/36 cells was described as elsewhere (Bian et al. 2017).
-
2-Dexoy-D-Glucose (2-DG), (L)-sodium lactate, 6-Aminonicotinamide (6-AN), ND-630, Etomoxir sodium salt, D-ribose 5-phosphate and Atglistatin (ATGL) were obtained from Topscience (Shanghai, China) and diluted in accordance with the instruction.
-
Neuro2a cells were seeded into 96-well plates at a density of 5 × 103/well and incubated with DMEM containing 2% FBS overnight. Cell culture medium then replaced with 2% FBS DMEM with different concentrations of inhibitors and incubated for 24 h. Then, 10 lL of Cell Counting Kit-8 (CCK-8, ThermoFisher, USA) solution were added to each well and incubated for 4 h. The absorbance of each well was measured at 450 nm using a microplate reader (BioTek, USA). All experiments were performed in quintuplicate.
-
Neuro2a cells with 80% confluence were infected with mock (virus suspension inactivated by heat at 56 ℃ for 30 min) or JEV at MOI of 0.1 for 2 h in serum-free DMEM. Then, the inoculum was removed and cells were washed twice with PBS. Cells were subsequently incubated in fresh DMEM containing 2% FBS and 1% antibiotics alone or added with different inhibitors at 37 ℃ for 24 h. The steps of primary neurons infection were the same as Neuro2a cell line, but Neurobasal medium (Gibco, New York, USA) was especially used for primary neurons.
The protocol of ATGL pre-treatment is as follows. Neuro2a cells were pre-treated with ATGL for 12 h before infection, then the medium containing ATGL was removed and cells were washed twice with PBS. Infection of JEV was then performed and subsequently incubated in fresh DMEM containing 2% FBS and 1% antibiotics for 24 h.
-
1 × 107 Neuro2a cells were seeded in 15 cm cell culture dishes until completely adherent. After JEV- or mockinfected for 24 h, the supernatants were discarded and the cells were washed three times with PBS and scraped. Harvested cells were immediately snap-frozen in liquid nitrogen and stored at -80 ℃ for measurement.
-
Six biological replicates in JEV- or mock-infected Neuro2a cells were set up for metabolomics detection. Extraction of metabolites and LC-MS/MS analysis were provided by Biotree Biotech (Shanghai, China).
The metabolites with the variable importance in the projection (VIP) > 1 and P-value of Student's t-test < 0.05 were considered as significantly changed. In pattern-specific analysis such as glucose metabolism, purine and pyrimidine metabolism and lipid metabolism, metabolites in positive ion mode (POS) and negative ion mode (NEG) were analyzed together. When a metabolite was detected both in POS and NEG, the one with higher MS2 score was picked.
-
Three biological replicates in JEV- or mock-infected Neuro2a were set up. Total RNA extraction, transcriptome sequencing and differential expression analysis were provided by Biotree Biotech (Shanghai, China). Genes with |Fold Change|≥ 1.5 and adjusted P-value < 0.05 were assigned as differentially expressed.
-
The P-value and fold change of both differential expressed genes and metabolites were collated and summarized for network analysis. The metabolite-transcript regulatory networks were visualized using Cytoscape v3.6.1 software (San Diego, CA, USA).
-
Lactate in Neuro2a cells infected with Mock and JEV at 24 hpi and 48 hpi was determined by Lactic Acid assay kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions.
-
Cellular oxygen consumption rate (OCR) was measured using Seahorse XF-24 Metabolic Flux analyzer (Seahorse biosciences, Agilent). 1 × 105 Neuro2a cells were seeded in XF24 24-well plate until adherent and then infected with mock or JEV for 24 h. Basal respiration was measured in the absence of 2 mmol/L pyruvate and 25 mmol/L glucose. After the assessment of OCR in basal condition, 1 μmol/L oligomycin, 0.75 μmol/L carbonylcyanide p-triflouromethoxyphenylhydrazone (FCCP) and 1 μmol/L rotenone were subsequentially added. All OCR values were normalized to cell number after the experiment. All results were averages of five biological replicates.
-
Total RNA from cells was extracted with Total RNA Kit I (Omega, USA) and used as the template for reverse transcription. cDNA was synthesized using Hifair® Ⅱ 1st Strand cDNA Synthesis Kit (Yeasen, Shanghai, China). qRT-PCR experiments were carried out using Hieff® qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) according to the manufacturer's instructions to measure transcripts or JEV RNA levels. The level of mRNA expression was normalized with β-actin. The sequences of primers are listed as supplementary information (Supplementary Table S1).
-
Cells were lysed with RIPA Lysis and Extraction Buffer (ThermoFisher, USA) containing PMSF (ThermoFisher, USA) protease inhibitor. The titer of total protein was quantified using Protein Reagent Assay BCA Kit (ThermoFisher, USA). Western blot analysis was carried out according to the standard method. Antibodies used in this research were as follows: rabbit anti-JEV NS3 (GeneTex, CA) at 1:3000, rabbit anti-HK2 (Proteintech, China) at 1:2000, rabbit anti-OGDH (Proteintech, China) at 1:2000, rabbit anti-b-tubulin (Proteintech, China) at 1:8000. The PVDF membranes were visualized by infrared imaging system (Odyssey, LI-COR, USA).
-
BHK-21 cells (2 × 105/well) were seeded into 12-well culture plates and incubated overnight. Supernatants of cells were removed and then, cells were washed twice with PBS. Serial tenfold diluted samples collected from Neuro2a or primary neurons were added and incubated at 37 ℃ for 2 h. After viral adsorption, cell monolayer was covered with overlay medium (25 mL 4 × DMEM, 25 mL ddH2O, 50 mL 4% methycellulose and 2 mL FBS) for 5 days. Then, the overlay medium was removed and cell monolayer was stained with 1% crystal violet.
-
Except for special statistical analysis of transcriptome and metabolome described above, other statistical analyses used GraphPad Prism version 8.1 (GraphPad, CA). Statistical comparation were made by Student's t-test. P-values < 0.05 were considered significant (*P < 0.05, **P < 0.01, and ***P < 0.001).
Cells and Virus
Reagents
Cell Viability Assay
Viral Infection and Drugs Treatment
Sample Collection for Metabolomic and Transcriptomic Analysis
Metabolites Extraction and LC-MS/MS Analysis
RNA Extraction and Transcriptome Sequencing
Integrated Network Analysis of Transcriptomics and Metabolomics
Assay for Lactate
Flux Analyses
Real-time RT-qPCR
Western Blot Analysis
Plaque-Forming Assay
Statistical Analysis
-
Since the level of JEV genome in Neuro2a cells at different time-points post of infection had been detected, the time point of 24 h (defined as the early stage of infection compared to 48 h in our research) was seen as the highefficiency replicative period and more favorable for the study of cellular metabolomics reprogramming induced by JEV infection (Supplementary Fig. S1). Multivariate pattern recognition analyses were conducted after original metabolomics data management to provide an overview of the metabolomic changes. Principle component analysis (PCA, Supplementary Fig. S2A, S2B), supervised orthogonal projections to latent structures-discriminant analysis (OPLS-DA, Supplementary Fig. S2C, S2D) and permutation test of OPLS-DA models (Supplementary Fig. S2E, S2F) were applied and suggested the distribution between JEV-infected group and mock-infected group was notably distinguished.
Differential metabolites were determined by the combination of the VIP value (> 1) in OPLS-DA model and the P-value (<0.05) from student's t-tests. A total of 69 differential metabolites in NEG and 58 in POS were identified by mass spectrum matching (MS2 matching in human metabolome database and Kyoto Encyclopedia of Genes and Genomes (KEGG) database). Compared to the mockinfected group, 25 metabolites were significantly upregulated and 44 metabolites down-regulated in NEG, and 24 metabolites were up-regulated and 34 metabolites were down-regulated respectively in POS. A volcano plot was performed for visualizing all differential metabolites detected between JEV and mock-infected Neuro2a cells (Fig. 1A, 1B).
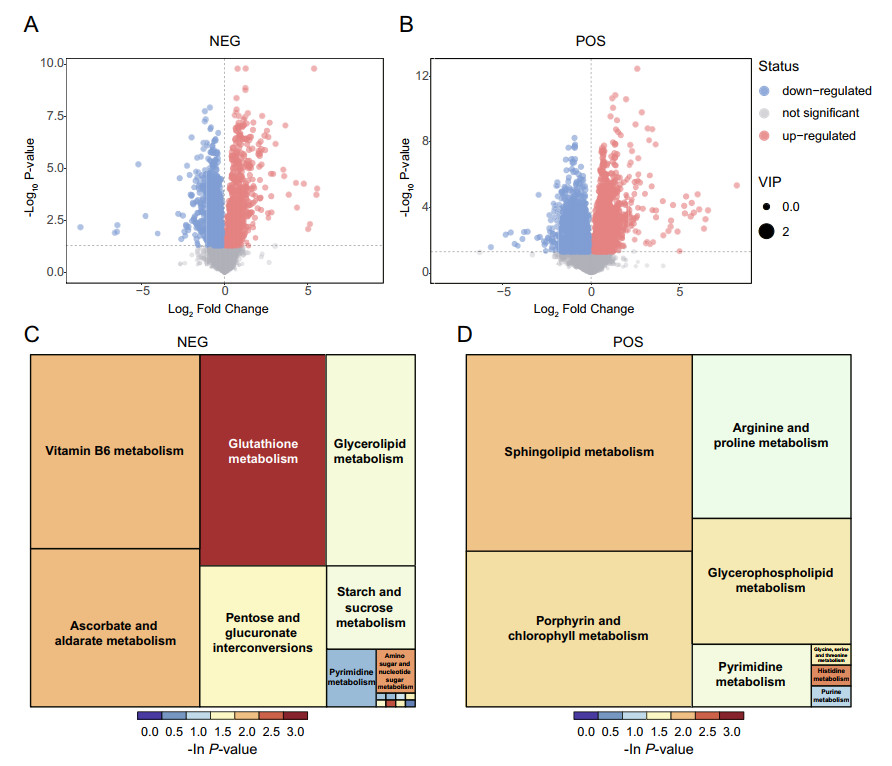
Figure 1. Differential metabolite identification and pathway enrichment analysis. Volcano plot for JEV- vs mock group in negative ion mode (NEG) (A) and positive ion mode (POS) (B). Each plot represents a metabolite identified. Significantly up-regulated metabolites were labeled as red points, while the down-regulated metabolites were marked as blue points. Treemap plot of pathway enrichment based on KEGG pathway analysis for JEV-infected group vs mock group in NEG (C) and POS (D). The area size of the square indicates the impact of the pathway in the topology analysis, and the color represents the P value of the enrichment analysis [take the negative natural logarithm, -ln(p)].
To explore the potential metabolic pathways affected by JEV infection, differential metabolites were annotated and assigned based on KEGG database (http://www.kegg.jp/kegg/pathway.html). The enrichment analysis of KEGG pathways included 16 pathways in NEG and 8 pathways in POS, specifically containing energy metabolism, material transportation, signal transduction, cell cycle regulation and other cellular physiological and biochemical processes. The KEGG enrichment treemap both in NEG (Fig. 1C) and POS (Fig. 1D) visualized all enriched pathways based on the number of enriched differential metabolites and P-value. Taking the treemap in NEG as an example, JEV infection mainly affects glutathione metabolism, vitamin B6 metabolism, glycerolipid metabolism and pyrimidine metabolism. Glutathione always plays a key role in the maintenance of cellular redox homeostasis. The decreased level of glutathione [fold change (FC) (JEV vs. mock) = 0.72], may be closely associated with neuron damage caused by oxidative stress during encephalitis. Pathways related to lipid metabolism, like glycerolipid metabolism in NEG, sphingolipid metabolism (sphingosine, FC = 0.53) and glycerophospholipid metabolism (choline, FC = 2.07) in POS, were also significantly enriched in KEGG pathway analysis. These results revealed that lipid metabolism was markedly modulated in JEV infected neuron cells and coincided with the findings of other flaviviruses like ZIKV and DENV (Martin-Acebes et al. 2016). The involvement of lipids in flavivirus biology can be from virion attachment, intracellular membrane remodeling, viral particle assembly interact with lipid droplet, to the regulation of cellular autophagy and apoptosis (Leier et al. 2018). Nucleotides as the basic materials both for cell proliferation and viral genome replication, were also obviously reprogrammed during JEV infection as evidenced by enriched purine and pyrimidine metabolism in NEG and POS displayed in Fig. 1.
Taken together, the metabolic reprogramming induced by JEV infection is reflected in a variety of biological functional pathways.
-
Genes with fragments per kilobase million (FPKM) value greater than 1 in the transcriptomics data were considered as expressed genes. Venn diagram (Fig. 2A) demonstrated the distribution of the number of genes annotated only in JEV-infected (568), mock-infected (547), or in both groups (11,832). Transcriptional changes in response to JEV infection were determined and the screening conditions for differentially expressed genes (DEGs) were |FoldChange| ≥ 1.5 and Padj (a common form of false discovery rate) < 0.05. A total of 4709 DEGs were identified based on the stringent statistical threshold, of which 2343 were up-regulated and 2366 were down-regulated (Fig. 2B). The top 20 most significantly enriched KEGG pathways both in up-regulated and down-regulated mode were selected to produce the scatter plot (Fig. 2C, 2D).

Figure 2. Transcriptome analysis in JEV and mock-infected Neuro2a cell line. A Venn diagram of differential expressed genes between JEV and mock group. B Volcano plot analysis using log2(fold change) as X-axis and -log10(Padj) as Y-axis. The up-regulated genes presented in red, whereas the down-regulated genes in green. Genes without significant statistic difference are presented in blue. The gray line shows the statistical significance cutoff used in this study. C– D KEGG pathway enrichment analysis. Up-regulated pathways were shown in (C), and down-regulated in (D). (Ordinate: the KEGG signal path; abscissa: gene ratio). Gene ratio is the ratio of the differentially expressed gene number in certain pathway to the total gene number in this pathway. The size of the dots represents gene ratio and the color of the dots represents enrichment significance
DEGs up-regulated in JEV-infected Neuro2a cells were mainly linked to pathways representing inflammatory processes including IL-17 signaling pathway, NF-kappa B signaling pathway, and TNF signaling pathway. The majority of down-regulated DEGs were involved in pathways associated with metabolism, especially the metabolism of lipid species. Biosynthesis of unsaturated fatty acids, sphingolipid metabolism, steroid biosynthesis and fatty acid metabolism were markedly down-regulated, which is somewhat different from other flaviviruses infection processes, where the lipid biosynthesis were mostly enhanced (Zhang et al. 2019b). This phenomenon indicated that JEV was more likely to prefer consuming lipid components within host cells, rather than promoting lipids biosynthesis to maintain virus proliferation in the early infection stage.
-
Through the specific-pattern analysis of metabolomics, we found that metabolites in the glycolysis pathway, such as pyruvate and lactate were all not significantly changed at 24 hpi (Fig. 3A). Duplicate test of cellular lactate was performed at 24 and 48 hpi subsequently for further validation. As shown in Fig. 3B, lactate contents were significantly increased with the increase of JEV infection time, which indirectly reflected enhanced glycolytic activity in JEV-infected Neuro2a cells. Thus, the content of pyruvate and lactate were not significantly changed in the early infection stage (24 hpi) may be due to their participation in the biosynthesis of multiple metabolites. Meanwhile, the level of malate, a metabolite near the end of the tricarboxylic acid (TCA) cycle, showed a statistically significant decreasing trend (FC = 0.70, P < 0.05).
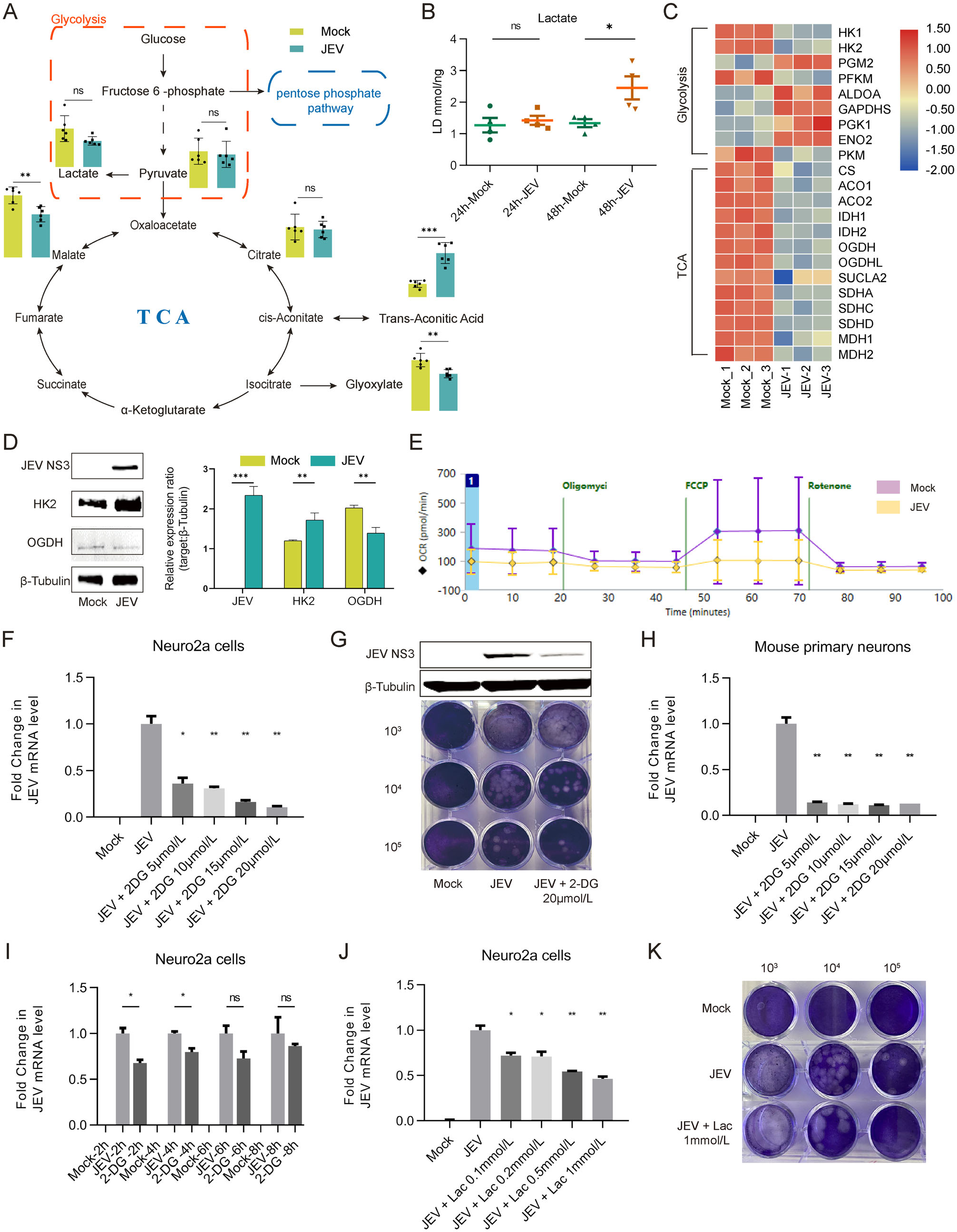
Figure 3. Glycolysis is essential for optimal replication of JEV. A Intermediates of glucose metabolism detected in Mock and JEV infected Neuro2a cells. B Lactate levels in Neuro2a cells were detected at 24 hpi and 48 hpi. C Heatmap analysis of expression levels of genes involved in glycolysis and TCA cycle. D Detection of JEV NS3, HK2 and OGDH proteins in Neuro2a cells infected with mock and JEV at 24 hpi. E Oxygen consumption rate (OCR) was detected both in JEV or Mock infected Neuro2a cells by Seahorse extracellular fluxanalyzer. F JEV mRNA levels in mock and JEVinfected Neuro2a cells treated with 2-DG at 24 hpi. G Detection of JEV NS3 protein level and infectious viral particles released in cell culture medium. H Viral mRNA levels in mock and JEV-infected mouse primary neurons treated with 2-DG at 24 hpi. The level of mRNA expression was normalized with β-actin. I Addition of 2-DG at various time-points post infection showed an important role of glycolysis in supporting the early steps of viral infection. J Viral mRNA levels in mock and JEV-infected Neuro2a cells treated with lactate at 24 hpi. K Exogenous addition of lactate could inhibit JEV replication in Neuro2a cells. 103, 104 and 105 represented the dilution rate. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
In addition, genes both involved in glycolysis and TCA cycle were analyzed and represented as a heatmap (Fig. 3C). The protein level of representative rate-limiting enzymes in the glycolysis pathway and TCA cycle were also detected in Neuro2a cells at 24 hpi (Fig. 3D). The expression level of hexokinase 2 (HK2), the first ratelimiting enzyme in the glycolysis pathway, was increased in JEV-infected Neuro2a cells. However, there is an inconsistency of HK2 mRNA level with protein level. We speculated that this inconsistency, on the one hand, might be due to the negative feedback of cumulative protein on its mRNA level, and on the other hand, due to the different degradation rate between protein and mRNA (Muller-McNicoll et al. 2019).
Meanwhile, the level of 2-oxoglutarate dehydrogenase (OGDH), a key enzyme in TCA cycle, was down-regulated. These results suggested that increased glycolysis and decreased OXPHOS in Neuro2a cells occurred during JEV infection. Then, oxygen consumption rate (OCR) was detected both in JEV- or mock-infected Neuro2a cells confirmed the impairment of OXPHOS under JEV infected condition (Fig. 3E).
Since glycolysis flux apparently increased during JEV infection, we employed 2-DG, a competitive inhibitor targeting hexokinase, to block glycolysis activity. Cell viability assay was performed in Neuro2a cell line treated with various concentrations of 2-DG and showed a noncytotoxic effect (Supplementary Fig. S3A). The results showed that 2-DG could effectively inhibit JEV replication both in Neuro2a cell line (Fig. 3F, 3G) and mouse primary neurons (Fig. 3H). Furthermore, the addition of 2-DG at various time-points post of infection showed that the inhibitory effect of 2-DG on JEV replication could only exert at the early stage of the viral life cycle (2 hpi and 4 hpi) and showed an important role of glycolysis in the early steps of viral infection (Fig. 3I). This result is consistent with norovirus infection in RAW cells (Passalacqua et al. 2019).
Since the content of lactate gradually accumulated with infection, we further evaluated its effect on JEV replication. Of note, addition of lactate into Neuro2a cells infected with JEV could effectively inhibit JEV replication (Fig. 3J, 3K). The possible reason might be that increased lactate provided negative feedback inhibition of glucose utilization and glycolysis activity (Brooks 2020). Taken together, these results demonstrated that glycolysis is essential for optimal JEV propagation.
-
Universally, PPP is a major branch pathway of glycolysis for de novo nucleotide synthesis. Although metabolites in this pathway had not changed significantly, the levels of some substances in downstream, such as metabolites associated with purine and pyrimidine metabolism were pronounced affected by JEV infection (Fig. 4A). Except for some metabolites in the early steps of purine synthesis such as AICAR, dIMP and D-ribose-1P, the level of other metabolites was mainly reduced upon JEV infection, mostly owing to the heavily reliance on host nucleotide intermediates for viral genome production. Since nucleotide metabolism plays a crucial role in viral replication, PPP inhibitor 6-Aminonicotinamide (6-AN) was applied to determine the effect of PPP on JEV infection. It is clear that PPP is of great importance in maintaining cellular physiological proliferation, its blocking will inevitably affect the cell's own replication process (Supplementary Fig. S3B). However, the inhibitory effect of 6-AN on JEV propagation is extremely obvious at the viral genome, protein expression and released infectious viral particles level both in Neuro2a cell line (Fig. 4B–4D) and mouse primary neurons (Fig. 4E–4G). On the one hand, this may be partly owing to the negative effect of 6-AN on cellular proliferation, and on the other hand, perhaps due to the inhibitory effect of 6-AN on nucleotides synthesis, which reduces nucleotide precursors required for viral genome replication. Furthermore, we supplemented D-ribose-5- phosphate, the final product of PPP and generally be used in the synthesis of nucleotides, under 6-AN treatment condition (Fig. 4H). The results showed that anaplerosis of D-ribose-5-phosphate could partially restore the replication of JEV and suggested the indispensable role of PPP in providing nucleotide precursors in JEV-infected neurons.
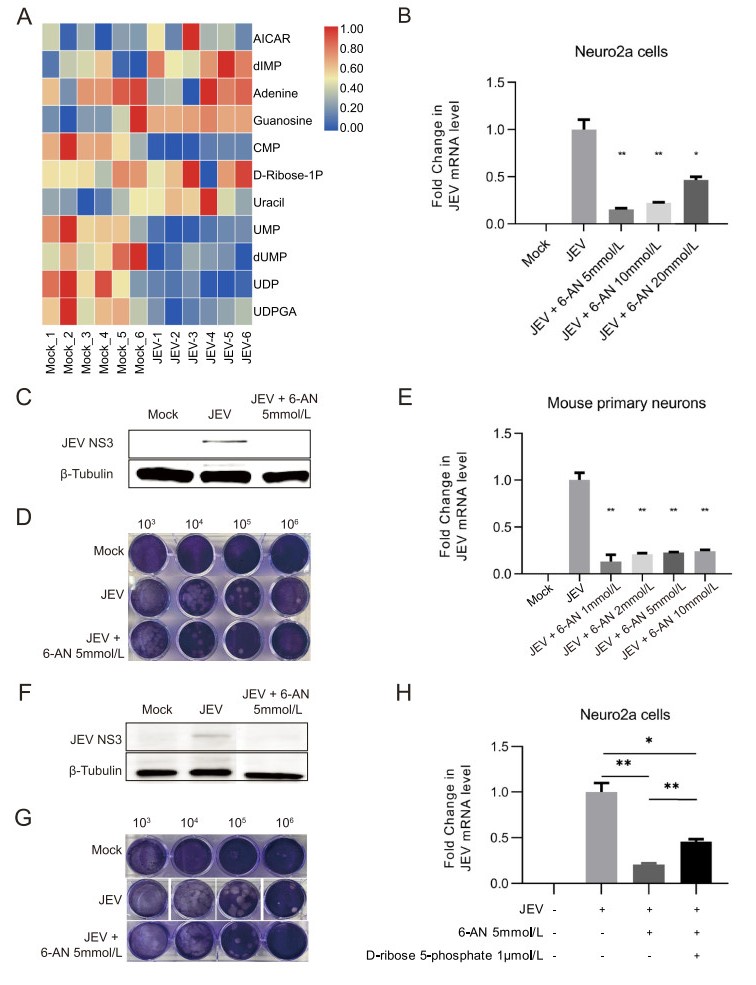
Figure 4. PPP is indispensable for JEV replication. A Heatmap analysis of significantly changed metabolites associated with purine and pyrimidine metabolism. B–G Intervention of PPP by 6-AN significantly inhibits JEV replication in Neuro2a cell line and mouse primary neurons at 24 hpi. JEV mRNA levels in JEV-infected Neuro2a cells (B) and mouse primary neurons (E) treated with 6-AN at 24 hpi were detected by qPCR analysis. The level of mRNA expression was normalized with β-actin. The expression levels of viral protein NS3 in JEV-infected Neuro2a cells (C) and mouse primary neurons (F) were detected by Western blot analysis. Plaque formation assay shows the reduction of plaque generation in JEV-infected Neuro2a cells (D) and mouse primary neurons (G). 103, 104, 105 and 106 represented the dilution rate. H qPCR analysis of JEV mRNA level shows that anaplerosis of D-ribose 5-phosphate under 6-AN treatment condition could partially restore the viral replication. *, P < 0.05; **, P < 0.01.
-
To further clarify the changes in the metabolic profile of Neuro2a cells during JEV infection, the metabolictranscriptomic regulatory network was constructed based on the integrated analysis of DEGs and differential metabolites identified in POS (Fig. 5A). (Since network analysis of NEG was similar to POS, the results were not exhibited here). The systematic network showed a complex correlation between metabolites and DEGs and, it is to be noted that lipid metabolism (red dotted box in Fig. 5A) was highly aggregated in the overall network analysis. It indicated that the anabolism and catabolism of lipid species were thoroughly reprogrammed in neurons during JEV infection. Network of Lipid-associated metabolites and DEGs were further performed and indicated that lipid metabolism might be the key biological process related to virus propagation and the development of encephalitis (Supplementary Fig. S4).
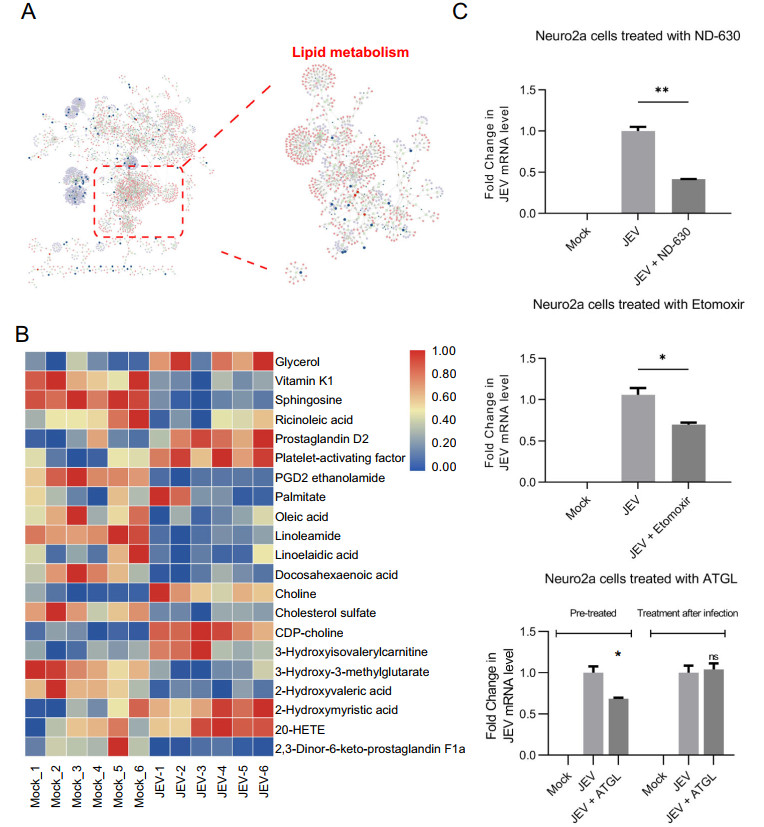
Figure 5. Reprogramming of lipid metabolism in neuro2a during JEV infection. A Integrated network analysis of metabolomic and transcriptomic profiling reveals lipid metabolism was significantly reshaped during JEV infection. B Heatmap showing relative levels of the lipids, sterols, and their derivatives. C The effect of lipid metabolism related inhibitors on JEV replication. JEV mRNA levels were detected by qPCR analysis. The level of mRNA expression was normalized with β-actin. ND- 630: 0.5 nmol/L; Extomoxir: 0.5 μmol/L; ATGL: 0.5 μmol/ L. *P < 0.05; **P < 0.01; ns, not significant.
Then, metabolites related to lipid metabolism were filtered and visualized as a heatmap to reveal the cellular lipid metabolism profile (Fig. 5B). Most of them displayed a downward trend after JEV infection, suggesting a heavy consumption of lipids to maintain virus replication. Meanwhile, genes which encode rate-limiting enzymes in fatty acid biosynthesis, such as fatty acid synthase (FASN, FC = -1.59) was also down-regulated at 24 hpi in transcriptomics data. This suggests that lipid species were mainly consumed by catabolism at the early stage of JEV infection.
It is noteworthy that metabolites within arachidonic acid metabolism, such as 20-hydroxyeicosatetraenoate (20- HETE, FC = 1.44) and prostaglandin D2 (PGD2, FC = 2.12) were significantly up-regulated in JEV-infected Neuro2a cells. Metabolites related to arachidonic acid metabolism generally served as pro-inflammatory mediators in promoting cellular oxidative stress. Thus, the increased level of 20-HETE and PGD2 might be partially responsible for the progression of encephalitis.
The level platelet-activating factor (PAF), which is involved in the increase of vascular permeability and the infiltration of leukocytes, was also markedly increased in JEV-infected Neuro2a cells. Previous studies on the model of herpes simplex virus type-1 (HSV-1) encephalitis have confirmed that PAF is closely involved in leukocytes infiltration and disease progression (Brooks 2020). The increased content level of PAF (FC = 2.35) in our metabolomics data might also play a similar role in encephalitis caused by JEV infection.
We next investigated whether lipid metabolism is required for optimal JEV infection by intervening host cell lipid metabolic process through several inhibitors (Fig. 5C). ND-630, an inhibitor of acetyl-CoA carboxylase (ACC) dimerization, was used to repress fatty acid synthesis and showed a potent antiviral effect in Neuro2a cells (cell viability assay seen in Supplementary Fig. S3C). Furthermore, interfering fatty acid beta-oxidation with carnitine palmitoyltransferase-I (CPT-1) inhibitor Etomoxir also exhibited an inhibitory effect on JEV replication (Cell viability assay seen in Supplementary Fig. S3D). Since lipid species were mainly catabolized within 24 h of JEV infection, lipolysis inhibitor ATGL was used to clarify the effect of lipolysis on JEV replication (No cytotoxicity when the concentration under 50 μmol/L (Mayer et al. 2013). It suggested that pretreatment of Neuro2a with ATGL for 12 h would significantly impede virus replication, while treatment after infection had no obvious effect.
Differential Metabolite Identification and Pathway Enrichment Analysis
Transcriptome Analysis of JEV Infected Neuro2a Cells
Glycolysis Is Essential for Optimal Replication of JEV
PPP is Indispensable for JEV Replication
JEV Rewires Cellular Lipid Metabolism
-
It is no wonder that viruses rely on host cell machinery to complete their entire life cycle. Intracellular metabolic status is an intrinsic factor that determines the successful viral infection and replication. Different viruses adopt diverse strategies to exploit host metabolism to optimize their replication (Thaker et al. 2019b; Thyrsted and Holm 2020). Currently, advanced techniques have been widely applied to reveal comprehensive interactions between virus and host in cellular metabolic facet, and then, explore potential antiviral tactics targeting virus-inducing metabolic re-programming.
Metabolomic approach is a common method for metabolic profile depicting in viral infection field. Central carbon metabolism comprised by glycolysis, PPP, and TCA cycle is predominant in maintaining a variety of cellular physiological functions and certainly vulnerable to be reprogrammed during viral infection. Metabolomic analyses of most DNA viruses, such as human cytomegalovirus (HCMV) (Rodriguez-Sanchez and Munger, 2019), EpsteinBarr virus (EBV) (Cai et al. 2017), and Kaposi's sarcomaassociated herpesvirus (KSHV) (Ma et al. 2015), suggest that central carbon metabolism has been reshaped by more similar approaches. The main manifestations are increased glucose uptake and glycolysis flux. In contrast to DNA viruses, metabolic manipulation strategies of RNA viruses are generally diverse (Mayer et al. 2019).
Take flaviviruses as examples, the global intracellular metabolic profiles of human foreskin fibroblasts suggest that central carbon metabolism, especially glycolytic pathway in glucose utilization, was significantly perturbed during DENV infection (Fontaine et al. 2015). Viral nonstructural protein NS1 could directly interact with glycolytic enzyme gluceralde-hyde-3-phosphate dehydrogenase (GAPDH) and lead to increased glycolytic flux (Allonso et al. 2015). However, the infection of ZIKV exhibits distinct effects on glucose metabolism in human versus mosquito cells (Thaker et al. 2019a). The infection of ZIKV will promote increased glucose utilization both in the TCA cycle and amino acid generation within human cells. In contrast, viral infection of C6/36 mosquito cells promotes glucose utilization via glycolysis and PPP, but decreases its contribution to the TCA cycle. Thus, dissimilar cellular outcomes with the cell death observed in ZIKV-infected human cells and survival observed in ZIKV-infected mosquito cells were caused. Different viruses usually have distinct metabolic requirements for their replication, and therefore exhaustive investigations are needed to reveal the metabolic interaction between certain pathogens and host cells.
Our study takes advantage of the integration of metabolomic and transcriptomic to unveil the global metabolic features in neuronal cells during JEV infection. Intermediates detected in central carbon metabolisms like lactate and malate suggested increased glucose contribution to glycolysis flux and decreased OXPHOS activity in JEV-infected Neuro2a cell line (Fig. 3A). Moreover, the expression levels of genes associated with glycolysis were also extremely different from TCA cycle, confirming the enhancement of glycolysis and the impairment of OXPHOS (Fig. 3B). It was consistent with the metabolic reprograming caused by most viruses previously reported. Host cells infected with viruses appear to display metabolic characteristics akin to the Warburg effect to satisfy the high bioenergetic and biosynthetic demands for virion production. One possible explanation is that increased flux of glycolysis and its branch PPP will be propitious to provide precursors for nucleotide synthesis and viral genome replication. In addition, lactate, the glycolytic product which plays an inhibitory role in cellular type I interferon response, will be accumulated with the enhancement of glycolysis (Zhang et al. 2019a). 2-DG and 6-AN were then employed to inhibit the glycolysis pathway and PPP. The results showed that both 2-DG and 6-AN could effectively inhibit JEV replication both in Neuro2a cell line and mouse primary neurons. These results indicated that glucose metabolism, both glycolysis and PPP are indispensable for the optimal propagation of JEV.
Lipidomic analyses of cells infected with flaviviruses have been well characterized over these years (Martin-Acebes et al. 2016, 2019). Conceptually, lipids are fatty acids and their derivatives, and substances biosynthetically or functionally related to these compounds (Fahy et al. 2011). Membrane-associated viral replication, particularly flaviviruses, is closely related to lipid synthesis, utilization, and transportation. It is generally known that the entry of flaviviruses depends on receptors located in lipid rafts for successful attachment. After internalization, the intracellular life cycle of flaviviruses is confined to the cytosol, thus remodeling cellular endomembranes into unique organellelike structures, which are defined as viral replication compartments (VRCs), is crucial for virus genome replication (Miller and Krijnse-Locker 2008). These VRCs can not only increase the local concentration of cellular and viral factors that are required for sufficient genome replication, but shield genomic RNA from cellular innate immune sensors. Owing to its multivariable lipid profiles, the structures, membrane curvatures, and permeabilities of VRCs vary greatly between different flaviviruses (Strating and van Kuppeveld 2017). Glycerophospholipids (GLs), including phosphatidyl-inositol (PI), phosphatidyl-serine (PS), phosphatidyl-choline (PC) and phosphatidyl-ethanolamine (PE), are the most abundant and important structural lipids in the eukaryotic cellular membrane. Viruses generally remodel GLs metabolism to form a suitable membranous compartment for successful infection and replication by changing its ratio. The downstream product PI (FC = 1.95) in GLs synthesis was elevated in JEV infection, demonstrating the enhancement of GLs biosynthesis. Another component of cellular membranes, PS, was significantly down-regulated in JEVinfected Neuro2a cells (FC = 0.36). The biological reason might be that PS often acts as an ''eat me'' signal that promotes the engulfment of apoptotic cells (Chen et al. 2015). In this way, flaviviruses might take advantage of this PSdependent uptake and infection termed as apoptotic mimicry mechanism (Mazzon and Mercer 2014). Therefore, mounts of PS might be used for the synthesis of mature viral envelope to mediate viral attachment and infusion. To be mentioned, the up-regulated of pro-inflammatory metabolites such as 20-HETE, PGD2 and PAF are particularly significant. These metabolites might be involved in the progression of encephalitis caused by JEV infection, thus partially inducing uncontrolled severe inflammation in brain (Hanna and Hafez 2018; Vilela et al. 2016).
Taken together, our study systematically described the metabolic landscape of neurons after JEV infection. Increased glycolysis flux and impaired OXPHOS in glucose utilization facilitate the biosynthesis of precursors needed for JEV replication. In addition, catabolic patterns of lipid metabolism were created to cope with the high anabolic demands of virion production and optimal virus propagation. Intervening host cell glucose metabolism and lipid metabolism by inhibitors showed potent antiviral effects and provided an auspicious therapeutic perspective on viral infectious diseases. This work provides further insights into the pathogenic mechanisms of JEV infection in the metabolic field and provides promising antiviral strategies by targeting metabolism.
-
This research was funded by the National Major Science and Technology Projects of China, grant number 2017ZX10202203-007-003.
-
JL and YL designed the experiments. ML and JY carried out the experiments and wrote the original draft. CY, XY, HZ, CL and ZX analyzed the data. JL and YL reviewed the manuscript and provided the financial support. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
















 DownLoad:
DownLoad: