HTML
-
Hepatitis B virus (HBV) infection remains a global threat to public health. Patients with chronic HBV infection are prone to cirrhosis, liver failure, and hepatocellular carcinoma (HCC) (Tang et al. 2018). In the past few decades, effective vaccines have been widely used to reduce the incidence of HBV infection, especially in newborns. However, the current therapeutic strategies for HBV infection using pegylated interferon and nucleos(t)ide analogues have limited efficiency against the disease (Liang et al. 2015; Subic and Zoulim 2018; Fanning et al. 2019).
HBV, which belongs to the Hepadnaviridae family, is an enveloped virus with a double-stranded DNA genome of a size of 3.2 kb, and is thus one of the smallest DNA viruses currently known (Karayiannis 2017). HBV replicates through reverse transcription (Glebe and Bremer 2013; Tong and Revill 2016). HBV covalently closed circular (ccc) DNA copies are formed in the nucleus, then serve as templates for viral transcription, and generate five major RNA molecules. These molecules include 3.5-kb pregenomic (pg) RNA and preC RNA, 2.4- and 2.1-kb preS/S mRNAs, and 0.7-kb HBx mRNA, which encode core/polymerase proteins, precore protein, large envelope protein, middle and small envelope proteins, and X protein, respectively (Gish et al. 2015; Nassal 2015). Subsequently, the core protein, pgRNA, and viral DNA polymerase are recruited to form nucleocapsids (NCs). In the process of virion release, NCs can directly be assembled with glycoprotein envelope and trigger the secretion of nascent virions, or be reintroduced into the nucleus for amplification of the cccDNA pool (Guo et al. 2007a; Kock et al. 2010). Noninfectious subviral particles (SVPs) composed of three envelope proteins, S-, M-, and L- HBV surface antigen (HBsAg), are independently secreted from host cells only through the endoplasmic reticulum (ER)-Golgi secretion pathway.
The life cycles of many viruses can be regulated by the mammalian target of rapamycin (mTOR) signaling pathway (Ranadheera et al. 2018; Bossler et al. 2019). The mTOR signaling pathway is a key regulator of many cellular processes including cell metabolism, growth, proliferation, survival and immunity (Laplante and Sabatini 2012; Liu Y et al. 2015; Saxton and Sabatini 2017), and is abnormally overactivated in numerous human cancers (Ciuffreda et al. 2010; Forbes et al. 2011). Moreover, the mTOR signaling pathway is activated during infection by many viruses, including HBV. Previous studies have shown that the interaction of mTOR signaling pathway and HBV life cycle is complex. While HBV proteins like the HBV X (HBx) protein are able to modulate this pathway (Zhu et al. 2017; Wang X et al. 2019). HBV replication and gene expression are also regulated by the mTOR signaling pathway (Guo et al. 2007b; Zhang et al. 2011; Huang et al. 2014; Li et al. 2015; Lin et al. 2017; Wang et al. 2020a, 2020b). In addition, the mTOR signaling pathway restricts or degrades viral particles and SVPs in a lysosome-dependent manner (Lin et al. 2019c; Wang et al. 2020b). In this review, we summarize and discuss the major findings in this field.
-
The mTOR protein is an evolutionarily conserved Ser/Thr kinase in the phosphoinositide 3-kinases (PI3K)-related kinase (PIKK) family. mTOR is the catalytic subunit of two complexes, mTORC1 and mTORC2 (Wullschleger et al. 2006); the localization of these complexes in different subcellular compartments affects their activation and function (Betz and Hall 2013).
mTORC1 is a rapamycin-sensitive companion of mTOR that acts as a sensor for growth factors, pressure, energy status, oxygen, and amino acids to control many major cellular processes, including glucose homeostasis, lipid synthesis, and autophagy (Fang et al. 2001; Kim et al. 2002; Hay and Sonenberg 2004). The heterodimer composed of tuberous sclerosis 1 (TSC1) and 2 (TSC2) is a key upstream regulator of mTORC1. The GTP-bound form of Ras homolog enriched in brain (Rheb) directly interacts with mTORC1 and strongly stimulates its kinase activity. However, TSC1/2 negatively regulates mTORC1 activity by reversing Rheb into its inactive GDP-bound state (Inoki et al. 2003a; Tee et al. 2003). Many upstream signals including growth factors that regulate mTORC1 through the PI3K and Ras pathways, are transmitted through the TSC1/2 complex. Effector kinases, including protein kinase B (PKB/Akt) (Chen et al. 2018), extracellular signal-regulated kinase 1/2 (ERK1/2) (Ma et al. 2005), and p90 ribosomal S6 kinase (RSK) (Roux et al. 2004), can directly phosphorylate and inactivate the TSC1/2 complex, thereby activating mTORC1. Amino acids also activate mTORC1. Under conditions with sufficient amino acid supply, mTORC1 is activated via localization with the Ragulator-Rag complex (RagA/B and RagC/D) on the lysosome surface after activating Rheb (Efeyan et al. 2012; Groenewoud and Zwartkruis 2013). The activation of mTORC1 can enhance protein or lipid synthesis by modulating the translation regulator p70 ribosomal S6 kinase 1 (S6K1) and the eukaryotic translation initiation factor 4E binding protein 1 (4EBP1) or sterol regulatory element-binding protein 1/2 (SREBP1/2) (Duvel et al. 2010; Wang et al. 2011; Guo et al. 2019), respectively. Han et al. proved that mTORC1 also regulates the trafficking and maturation of SREBP1 through CREB-regulated transcription coactivator 2 (CRTC2). Under nutrient-rich conditions or insulin stimulation, mTOR activation leads to the phosphorylation and activation of SREBP1, thereby enhancing lipogenesis (Han et al. 2015).
The signaling pathway triggered by mTOR competes with other regulators of cellular metabolisms, such as AMP-activated protein kinase (AMPK), another major metabolic sensor. AMPK is activated in response to the increased AMP/ATP ratio, for example, after starvation of glucose. Importantly, AMPK directly or indirectly inhibits mTOR activity (Inoki et al. 2003b; Gwinn et al. 2008). Mitochondria biogenesis and turnover mediated by mTOR and AMPK are essential for regulating the cellular metabolism, especially mitochondria. Peroxisome proliferator-activated receptor (PPAR) gamma coactivator 1α (PGC1α) is a nuclear cofactor that plays a key role in mitochondrial biogenesis, oxidative metabolism, and gluconeogenesis. A report showed that nuclear mTORC1 controls the transcriptional activity of PGC1α by changing its physical interaction with another transcription factor Yin Yang 1 (YY1) in skeletal muscle (Cunningham et al. 2007). However, AMPK directly phosphorylates and activates PGC1α in skeletal muscle (Jager et al. 2007). Additionally, under glucose starvation conditions, inactivation of mTORC1 is accompanied with increased expression of PGC1α in hepatoma cells (Wang et al. 2020a). Consistent with this finding, mTORC1 reduces the fasting-induced activation of PPARα (Sengupta et al. 2010).
In addition, mTORC1 enhances glycolysis and glucose uptake by regulating the transcription factor hypoxia-inducible factor (HIF1) (Semenza 2010) and promotes cell growth by negatively regulating autophagy, which is necessary for the recycling of damaged organelles and the adaptation of organisms to nutrient starvation (Kim et al. 2011). In mammals, mTORC1 directly phosphorylates and inhibits the ULK1/Atg13/FIP200 complex, which is an essential kinase complex that initiates autophagy. Activating mTORC1 can inhibit lysosome function by inhibiting the activity of transcription factor EB (TFEB), the master regulator of lysosomal biogenesis. Nutritional starvation or inhibition of mTORC1 activates TFEB by promoting its nuclear translocation, thereby promoting autophagic flux (Zhou et al. 2013).
On the other hand, mTORC2 is a rapamycin-insensitive companion of mTOR (Jacinto et al. 2004; Sarbassov et al. 2004) and can be directly activated by PI3K (Shimobayashi and Hall 2014). Interestingly, long-term rapamycin treatment can also inhibit mTORC2 in some cell types, which may be caused by inhibition of mTORC1 and reduction in binding of mTORC1 to the nascent mTORC2 complex (Delgoffe et al. 2011). Compared with the mTORC1 pathway, the mTORC2 pathway is much less understood. The downstream genes of mTORC2 include several members of the AGC kinase subfamily, such as Akt, serum and glucocorticoid-induced protein kinase 1 (SGK1) and protein kinase C-α (PKC-α). mTORC2 directly activates Akt by phosphorylating the hydrophobic motif (Ser473) of Akt, which is required for the maximum activation of Akt (Sarbassov et al. 2005). Akt regulates cellular metabolism, survival, apoptosis, growth and proliferation through phosphorylation of several effectors.
-
mTOR signaling is a key regulator of many cellular processes including metabolism, proliferation and survival. As a consequence, the life cycles of numerous viruses are regulated by the mTOR signaling pathway (Ranadheera et al. 2018; Bossler et al. 2019). Notably, chronic HBV infection is one of the key factors in the development of HCC. Guo et al. demonstrated that HBV RNA transcription and subsequent DNA replication are inhibited by Akt, an upstream factor of mTORC1, in HBV-transfected cells (Guo et al. 2007b). These inhibitory effects appear to be mediated by mTORC1 activation because this inhibition can be reversed by rapamycin. In addition, PI3K, Akt and mTOR inhibition using high concentrations of drugs can promote HBV RNA transcription and DNA replication in vitro. Consistent with these findings, HBsAg expression in chronic hepatitis B is significantly upregulated compared with that in HBV-associated HCC (Wang M et al. 2013), which has a higher PI3K/Akt activity. However, low inhibitor concentrations of PI3K, Akt and mTOR mainly modulate posttranscriptional steps of HBV life cycle (Lin et al. 2020). The roles of HBV in the mTOR signaling pathway are summarized in Fig. 1.
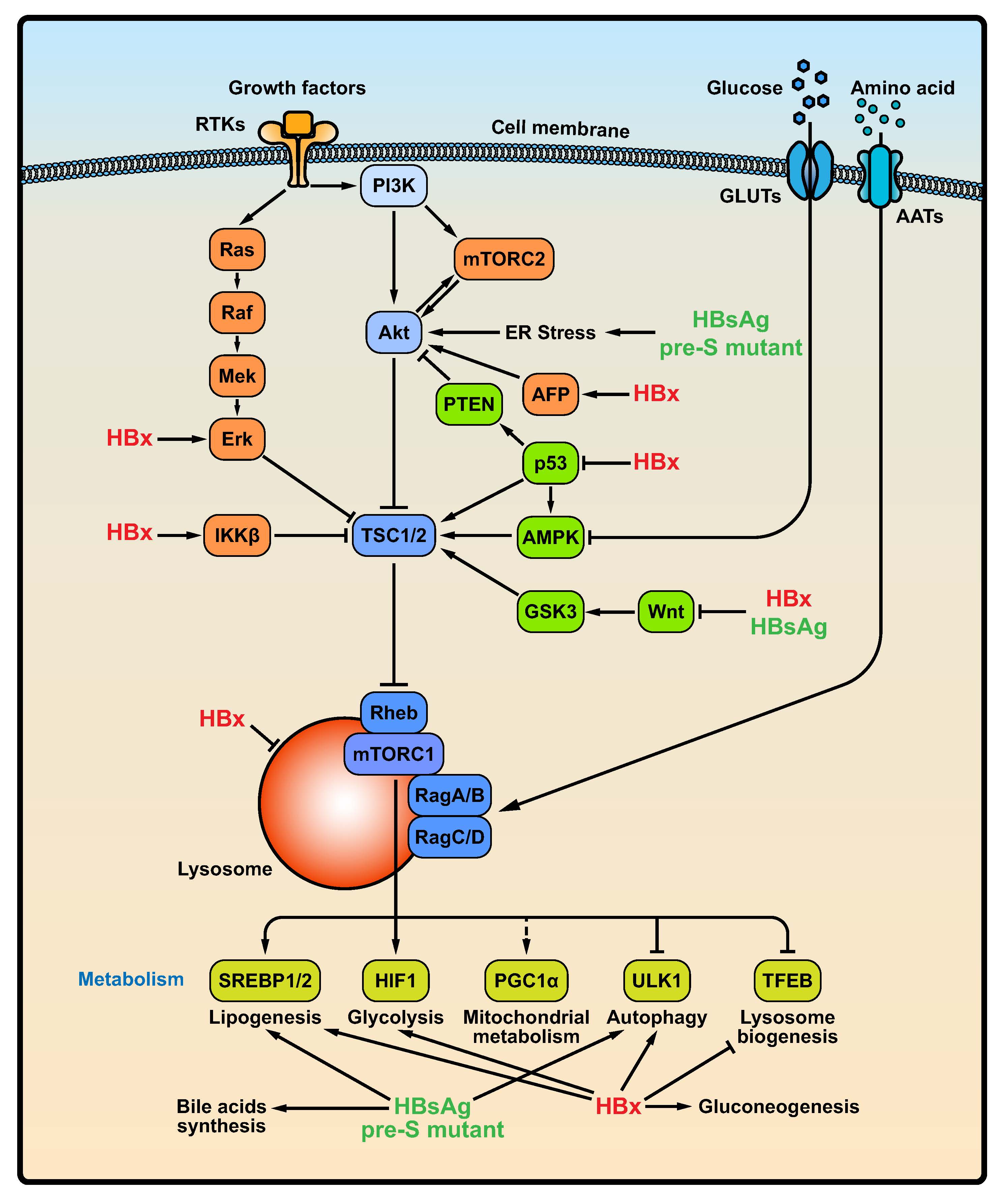
Figure 1. HBV infection activates the PI3K/Akt/mTOR signaling pathway. Growth factors and nutrition, including glucose and amino acids, are factors known to activate the PI3K/Akt/mTOR signaling pathway. During the HBV infection process, HBsAg and pre-S mutant protein may accumulate in the ER lumen to trigger ER stress, thereby activating the PI3K/Akt/mTOR signaling pathway. In addition, HBsAg and HBx indirectly activates the mTOR signaling by regulating Wnt/β-GSK3 pathway. HBx-mediated activation of the RAS/RAF/MAPK pathway and IKKβ induces the mTOR signaling pathway by inhibiting the activity of TSC1/2. Additionally, HBx indirectly modulates p53, AFP, or Wnt/β-GSK3 pathway to activate the mTOR signaling pathway. More importantly, HBV infection interferes with hepatic metabolism signaling pathways, including glucose homeostasis and lipid metabolism pathways. Abbreviations: GLUTs, glucose transporters; AATs, amino acid transports; RTKs: receptor tyrosine kinases; IKKβ, IκB kinase β; AFP, alpha fetoprotein; PTEN, phosphatase and tensin homolog; SREBP1/2, sterol regulatory element-binding protein 1/2; PGC1α, PPARγ coactivator 1α; HIF1, hypoxia-inducible factor; TFEB, transcription factor EB.
-
The HBV entry process may activate the Akt/mTOR signaling pathway (Xiang and Wang 2018). However, short-term treatment with Akt inhibitors does not block HBV entry, suggesting that Akt activation induced by HBV infection is not essential for the viral entry process. Yet, it is not clear how the activation of the Akt/mTOR pathway is triggered. It is reported that uptake of various ligands or virus particles may activate the cellular Akt/mTOR pathways (Ji and Liu 2008; Eaton et al. 2014). This process needs to be examined in the future. HBV virions and noninfectious SVPs contain three glycosylated HBV surface antigens, S-, M-, and L-HBsAg (Mehta et al. 1997; Sigrid Schmitt 1999). HBV infection can induce the synthesis of a large quantity of viral proteins and lead to the accumulation of HBsAg in the ER lumen to trigger ER stress, thereby activating the PI3K/Akt/mTOR pathway (Choi et al. 2019). L-HBsAg has been shown to activate the PI3K/Akt/mTOR pathway to promote tumorigenesis (Liu et al. 2011). Consistent with this evidence, Teng et al. reported that wild type or mutant pre-S proteins can activate mTOR in Huh7 cells (Teng et al. 2011). It has also been reported that pre-S1 deletions lead to accumulation of L-HBsAg, thereby triggering ER stress to activate the mTOR signaling pathway (Choi et al. 2019). The pre-S mutant proteins can induce ER stress, leading to formation of ground glass hepatocytes (GGHs) (Wang et al. 2003). In addition, L-HBsAg and pre-S2 mutants activate the mTOR signaling pathway through induction of ER stress-dependent vascular endothelial growth factor (VEGF) A and Akt activation, resulting in aberrant glucose uptake and lactate production in tumorigenic processes. In accordance with this finding, the pre-S2 mutant proteins also trigger ER stress-mediated VEGF/Akt/mTOR and NF-κB/COX-2 signaling pathways to induce cell inflammation and transformation (Hung et al. 2004; Yang et al. 2009). Moreover, pre-S mutant-induced ER stress may also modulate the activity of SREBP1, ATP citrate lyase (ACLY), and fatty acid desaturase 2 (FADS2) (Teng et al. 2015b), major regulators of lipid metabolism, by activating the Akt/mTOR signaling pathway (Yang et al. 2009). Interestingly, activation of mTOR can inhibit HBsAg synthesis by promoting the interaction between histone deacetylase 1 (HDAC1) and the transcription factor YY1, which binds to the pre-S1 promoter (Teng et al. 2011). Disruption of HDAC1 eliminates the inhibitory effect of mTOR on pre-S transcription.
Collectively, the evidence indicates that wild type and mutant HBsAg accumulation can trigger ER stress and activate the Akt/mTOR signaling pathway. This process may promote tumorigenesis and is considered as an underlying mechanism of HBV-associated hepatocarcinogenesis. However, whether and how HBV may benefit from the HBsAg-induced ER stress remains elusive. Our recent approach using low doses of tunicamycin, a glycosylation inhibitor, showed that even artificially triggered ER-stress strongly promotes HBV replication and production by modulating viral assembly and release (unpublished data).
-
The HBx protein, the product of the smallest of the four overlapping open reading frames of the HBV genome, plays a vital role in HBV replication and the pathogenesis of HBV-associated HCC. HBx transfection in hepatoma cells increases the expression of mTOR (Wang P et al. 2013), and enhance the formation of autophagosomes and autolysosomes through the PI3K/Akt/mTOR signaling pathway (Wang P et al. 2013; Wang et al. 2014). HBX may also mediate the RAS/RAF/MAPK pathway activation, thereby inducing the mTOR signaling pathway by inhibiting the activity of TSC1/2 (Tarn et al. 2001; Chung et al. 2004). In addition, mTOR activation by HBx appears to be dependent on IκB kinase β (IKKβ), a major downstream kinase in the TNFα signaling pathway. IKKβ physically interacts with and phosphorylates TSC1 at Ser487 and Ser511, which results in TSC1 suppression and consequent mTOR activation (Lee et al. 2007). In addition, in an HBx transgenic (Tg) mouse model and HBV-associated HCC hepatocytes, HBx expression can regulate the IKKβ/mTOR/S6K1 signaling pathway (Yen et al. 2012). Disruption of IKKβ reverses HBx-mediated S6K1 activation, cell proliferation and VEGF production. Furthermore, HBx upregulates the expression of AFPR and AFP, which are early indicators of HBx-driven hepatocarcinogenesis, to activate the PI3K/Akt/mTOR signaling pathway (Zhu et al. 2015, 2017). Moreover, the HBx-induced antiapoptotic mechanism is essential for promoting the malignant transformation of hepatocytes via activation of the PI3K/Akt/mTOR signaling pathway (Wang X et al. 2019). The weakness of these studies is that the expression levels of HBx protein in transfected cells may be higher than that during HBV infection and replication and cause non-physiological effects.
The existing evidence suggests that HBx binds to and inactivates the transcription factor and tumor suppressor p53. Chung et al. reported that HBx disrupts p53-mediated transcription of PTEN, which is a negative regulator of the PI3K/Akt/mTOR signaling pathway (Chung et al. 2003). Consistent with this report, HBx can inhibit the expression (Lee and Rho 2000) or change the promoter binding site of p53 (Chan et al. 2013, 2016), which has been proven to inhibit the mTOR signaling pathway via activation of AMPK or TSC1/2 (Feng et al. 2005). Moreover, HBV proteins, including HBsAg and HBx, activate Wnt/β-catenin pathway (Cha et al. 2004; Lee et al. 2005; Daud et al. 2017) to inhibit GSK3 and TSC2, thereby promoting the mTOR signaling pathway (Inoki et al. 2006).
Altogether, the evidence indicates that HBx may indirectly activate the PI3K/Akt/mTOR signaling pathway, which plays a vital role in the development and progression of HBV-associated HCC. Though the reports mentioned above indicate an important role of HBx in the activation of mTOR signaling pathway, the findings are rather diverse and could not be integrated into a clearly unified concept. Future studies are required to find the connections between the events described in these studies.
-
The liver is an important organ for metabolic processes and plays key roles in glucose homeostasis and lipid metabolism. During HBV infection, many cellular signal transduction pathways are altered, including the PI3K/Akt/mTOR pathway (Teng et al. 2011, 2015a; Xu et al. 2013; Rawat and Bouchard 2015). The mTOR signaling pathway not only plays an essential role in coordinating anabolism and catabolism at the cellular level, but also has an important function in metabolic regulation in organisms (Saxton and Sabatini 2017).
With the discovery of a major bile salt carrier human sodium taurocholate cotransport peptide (hNTCP) as a functional receptor for HBV, the role of HBV in cellular metabolism has been placed at the forefront. Yan et al. showed that HBV pre-S1 may interfere with the physiological function of hNTCP to block bile acid uptake (Yan et al. 2014; Cheng et al. 2019). Decreased uptake of bile acid could promote compensatory bile acids synthesis and increase cholesterol supply to maintain homeostasis (Geier 2014; Patman 2014). In a transplant mouse model with HBV infection, an increased level of human cholesterol 7α-hydroxylase (CYP7A1), the rate-limiting enzyme that converts cholesterol to bile acids, was shown, consistent with decreased nuclear translocation of farnesoid X receptor (FXR, the positive transcription factor of SHP), and significantly reduced levels of SHP, the corepressor of hCYP7A1 transcription (Oehler et al. 2014).
HBV infection has been shown to regulate gluconeogenesis and aerobic oxidation of glucose. Park et al. showed that HBx acts as a positive regulator of gluconeogenesis (Shin et al. 2011). Increased HBx expression upregulates the expression of the key gluconeogenesis enzymes PEPCK and G6Pase and the production of hepatic glucose, leading to hyperglycemia and impaired glucose tolerance in HBx Tg mice. In addition, HBV pre-S2 mutants can induce aerobic oxidation of glucose by activating the mTOR signaling pathway (Teng et al. 2015a). HBV infection stimulates the expression of G6PD, the first and rate-limiting enzyme of the pentose phosphate pathway (PPP), through HBx-mediated nuclear factor erythroid 2-related factor 2 (Nrf2) activation (Liu B et al. 2015).
In addition, mTORC1 activation stimulates de novo lipogenesis by interacting with PPARγ (Li et al. 2014) and regulating the activity of SREBP1, two master regulators of lipid metabolism in hepatocytes (Peterson et al. 2011). It has been reported that HBx expression leads to lipid accumulation in hepatocytes mediated by SREBP1 and PPARγ (Kim et al. 2007). A number of studies have consistently demonstrated that HBV infection has an impact on lipid metabolism. Fatty acid binding protein 5 (Fabp5), acyl-CoA binding protein (ACBP), SREBP2, ACLY, and fatty acid synthase (FAS), which are involved in fatty acid metabolism and synthesis, are strongly upregulated in HBx Tg mice (Yang et al. 2008). HBV is an enveloped virus, and its successful assembly and secretion depend on the biogenesis of lipids and the formation of lipid membranes. HBx mediates the transcription of SREBP1, FAS, and PPARγ by activating lipogenesis induced by the nuclear receptor LXRα in HBV-associated HCC (Kim et al. 2008; Na et al. 2009). Furthermore, pre-S2 mutant-mediated induction of mTOR can activate SREBP1/ACLY/FAS, thereby inducing lipid accumulation (Teng et al. 2015b). On the other hand, HBV infection is associated with reduced prevalence of fatty liver, hypertriglyceridemia and metabolic syndrome in patients (Wong et al. 2012). This observation may have different interpretations and needs to be analyzed further.
Taken together, HBV infection interferes with hepatic metabolism pathways, including glucose homeostasis and bile acid and lipid metabolism pathways, and eventually leads to metabolic disorders. Alteration of these metabolic pathways may also contribute to the pathological process of HBV-associated HCC. On the other hand, viral infections require active metabolisms to supply energy and building blocks (Claus and Liebert 2014). HBV life cycle is also strongly dependent on host cell metabolism and its regulation (also see Conclusion). There are still many aspects in this direction for future research, for example, how different metabolic pathways and metabolites influence HBV replication via mTOR signaling pathways. Therefore, studies on the metabolic pathways during HBV infection could provide new insights and therapeutic approaches.
-
The activation of mTOR by HBV infection can enhance cellular metabolism, autophagy, and immune responses to reduce the damage to host cells. Although the PI3K/Akt/mTOR pathway benefits most viruses, the PI3K/Akt/mTOR pathway has been shown to reduce HBV replication in some experimental conditions and may be partly responsible for low or absent HBV replication in transformed and tumor cells. Specifically, triggering the PI3K/Akt/mTOR signaling pathway directly inhibits the transcription of HBV 3.5-kb and 2.4-kb RNA and HBV replication in hepatoma cells (Guo et al. 2007b). In addition, mTOR can inhibit HBV replication by preventing the recruitment of the YY1-HDAC1 complex to the pre-S1 promoter (Teng et al. 2011). However, the interaction between HBV replication and mTOR-regulated cellular processes is far more complex.
The mTOR kinase complex negatively regulates autophagy initiation and its inhibition activates the ULK kinase complex association with the PIK3C3/BECN1/ATG14 complex, leading to the formation of autophagosomes. Then, autophagosomes fuse with lysosomes to degrade cytoplasmic cargo (Fig. 2, bottom), whose activity also negatively regulated by the mTOR kinase (Lin et al. 2019c; Wang et al. 2021). Studies have found that autophagosome formation is essential for efficient HBV replication in different cell and animal model (Sir et al. 2010; Li et al. 2011; Tian et al. 2011; Lin et al. 2019a; Lin et al. 2019b; Lin et al. 2019c; Wang J et al. 2019; Wang et al. 2020a; Wang et al. 2020b). In contrast, autophagy inhibition can prevent HBV replication both in vivo and in vitro.
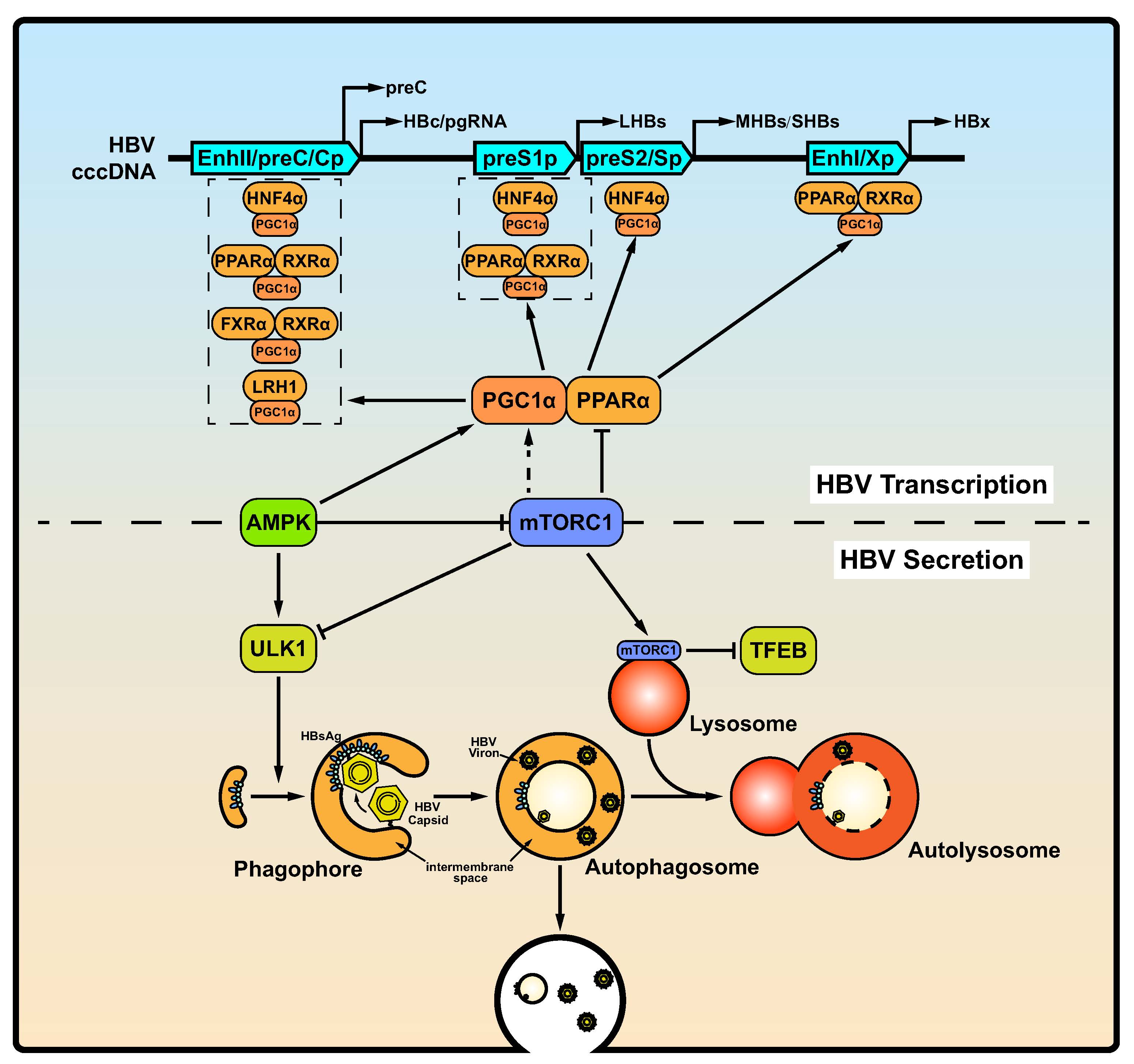
Figure 2. mTOR-mediated host responses regulate HBV RNA transcription, assembly and secretion of virions and subviral particles. Top: mTOR and AMPK, two major metabolic sensors, control the activity of PPARα and PGC1α to regulate HBV transcription through modulating HBV enhancers and promoters. Bottom: mTOR inhibits both the early (autophagosome formation) and late (lysosomal activity) phases of autophagy. HBsAg utilizes early autophagic structures for envelope formation. HBV virions and subviral particles are degraded during the late phase of autophagy. Abbreviations: PPARα, peroxisome proliferator-activated receptor alfa; PGC1α, PPARγ coactivator 1α; FXRα, farnesoid X receptorα; RXR, retinoid X receptor alpha; LRH1, liver receptor homolog 1; HNF4α, hepatocyte nuclear factor 4α; TFEB, transcription factor EB.
Recent studies demonstrated that HBV replication and assembly can be promoted by initiating the early phase of autophagy. However, a great part of HBV antigens and virions is degraded during the late phase of autophagy. Sir et al. showed that HBV DNA replication is markedly reduced by the PtdIns3K inhibitor 3-methyladenine (3-MA) or siRNA that targets the PtdIns3K or ATG7 gene to disrupt the initiation of autophagy (Sir et al. 2010). The noncoding microRNA-99 family targets Akt and mTOR to induce autophagy, thereby promoting HBV replication (Lin et al. 2017). Consistent with these findings, our previous study found that treatment with glucosamine (Lin et al. 2019c) or disruption of O-GlcNAcylation by OSMI-1 or siRNA that targets OGT greatly enhances HBV replication by initiating the early phase of autophagy by inhibiting mTOR signaling pathway (Wang et al. 2020b). Moreover, autophagy is initiated by inhibition of the Akt/mTOR signaling pathway at low glucose concentrations, with enhanced HBV replication at the same time (Wang et al. 2020a). Our recent study demonstrated that HBV inhibited lysosomal activity is restored to enhance HBV antigens degradation by inhibiting Akt/mTOR signaling after silencing CCDC88A in hepatoma cells with HBV replication (Wang et al. 2021). Some studies have reported that certain drugs, such as rapamycin (an mTOR inhibitor), cisplatin and dexamethasone, induce the initiation of autophagy and promote HBV replication by inhibiting the mTOR signaling pathway (Huang et al. 2014; He et al. 2018; Chen et al. 2019).
The critical role of autophagy in HBV replication has not only been demonstrated in HBV cell culture models but also in vivo. Tian et al. showed that the serum HBV DNA levels are significantly reduced and the HBV DNA replication intermediates were almost undetectable in HBV transgenic mice if the Atg5 gene is specifically knocked out in the liver (Tian et al. 2011). Our recent study illustrated that glucosamine increases HBV replication by blocking mTOR signaling via a feedback mechanism in a HBV mouse model (Lin et al. 2019c).
To maintain effective autophagic flux, autophagosome formation is linked with autophagosomal-lysosomal fusion and lysosomal degradation activity. Although mTOR regulates both early (autophagosome formation) and late (lysosomal activity) phase of autophagy, HBV assembly and release through enhanced autophagy are still beneficial for the production HBV particles despite the degradation by lysosomes. Recent findings also showed that HBV replication can interfere with the lysosomal functions and thereby evade the autophagic degradation process (Xie et al. 2016; Lin et al. 2019a, 2019b). A part of autophagosomes may not fuse with lysosomes for degradation, but rather release HBV proteins and virions through other pathways, such as fusing with MVBs (Wang et al. 2021) or exosomes as indicated by our unpublished work.
As mTOR pathway integrates many different signals, indirect crosstalk with some other major pathways may occur, thereby regulating downstream processes and HBV replication. For example, type I interferon signaling has been shown to crosstalk with Akt/mTOR signaling (Uddin et al. 1995; Lekmine et al. 2004). Our unpublished data revealed that type I interferon may modulate autophagy and subsequently HBV replication via Akt/mTOR pathway.
-
In this review, we have summarized recent progress from studies on the interaction between the cellular mTOR signaling pathway and HBV replication. mTOR first attracted the attention of researchers because it may play important roles in the development of HBV-associated HCC. The mTOR inhibitors are able to suppress cell growth in vitro and in vivo. Viral infections often alter host cellular metabolisms through regulating various cellular factors and pathways, including the PI3K/Akt/mTOR signaling pathway. The mTOR signaling pathway is essential in hepatocyte glucose and lipid metabolism, which are two important biological processes for efficient HBV replication. Additionally, mTOR negatively regulates autophagosome formation by inactivating the ULK kinase complex bound to the PIK3C3/BECN1/ATG14 complex and thereby reducing HBV assembly and release through autophagy. Up to date, the mTOR-mediated signaling pathway has been shown to be essential for HBV replication and pathogenesis in cell culture models. However, only few studies have been conducted in human tissues or animal models and need to be continued in the future. In addition, there are limitations in studying the cross-regulation between HBV infection and mTOR signaling in human tissues or animal models. The development of new technologies in molecular and cell biology will help to better understand the cross-regulation between HBV infection and mTOR signaling. In the future, this may help identify new therapeutic targeting.
Different concentrations of PI3K, Akt and mTOR inhibitors have different effects on HBV transcription and replication. Guo et al. showed that treatment of HBV-expressing HepG2.2.15 cells with high concentrations of inhibitors of PI3K, Akt, and mTOR (10, 5, and 10 μmol/L, respectively) increased the transcription of 3.5-kb and 2.4-kb viral RNA as well as the replication of HBV DNA (Guo et al. 2007b). However, some other studies including ours showed that low concentrations (≤ 1 μmol/L) of the inhibitors significantly enhanced HBV replication through enhancing autophagy instead of HBV transcription (Huang et al. 2014; Lin et al. 2020). There are many binding sites of ubiquitous and hepatocyte-enriched transcription factors in the promoter and enhancer regions of the HBV genome, including that for the transcription factors PGC1α and the PPARα/retinoid X receptor alpha (RXRα) complex. Previously evidences suggested that mTOR could regulate the activity of PGC1α and PPAR in vitro or in vivo (Raney et al. 1997; Shlomai et al. 2006; Shlomai and Shaul 2009; Reese et al. 2011; Weng et al. 2014), which play essential roles in HBV transcription. Moreover, PGC1α can be recruited by nuclear receptors (Fig. 2, top), including hepatocyte nuclear factor 4α (HNF4α), the PPARα/RXRα heterodimer, the FXRα/RXRα heterodimer, and liver receptor homolog 1 (LRH1), which positively regulate HBV pgRNA synthesis to enhance HBV replication (Reese et al. 2011; Weng et al. 2014). Therefore, high-concentrations of PI3K, Akt, and mTOR inhibitors may have a stronger regulatory effect on these transcription factors, while low-concentrations of these inhibitors mainly regulate the mTOR-induced autophagy pathway and have lower regulatory effects on these transcription factors. However, this hypothesis needs to be tested in future experimental approaches. It is likely that these nutrient-regulated transcription factors on HBV transcription also are regulated by HBV activated mTORC1.
Recently, increasing evidence has indicated that the mTOR signaling pathway plays multiple roles in immunity (Weichhart et al. 2015). The interaction of S6K and STING can regulate the activation of IRF3 to induce innate immunity (Wang et al. 2016). The mTOR signaling pathway can regulate c-Fos expression (He et al. 2015), and IFNα/β production (Cao et al. 2008). Besides, Delgoffe et al. reported that naïve T cells fail to differentiate into Th1, and Th17 cells in mTOR or Rheb gene knockout mice (Delgoffe et al. 2009, 2011). In addition, rapamycin at a high dose can attenuate CD8+ T cell responses (Pearce et al. 2009), and exerts immunostimulatory effects on memory CD8+ T cell differentiation (Araki et al. 2010). Thus, mTOR is involved in the regulation of both innate and adaptive immunity. It is tempting to speculate that mTOR-mediated innate or adaptive immunity may contribute to control HBV infection. Importantly, further understanding of the immunity and mTOR signaling may provide novel targets for regulating immunity and treating HBV-related disease.
Despite recent advances in drug discovery and development, effective antiviral drugs against HBV are still very limited, especially for the cure of chronic HBV infection. Thus, the development of effective, well-tolerated and affordable antiviral treatments is necessary and urgent. The HBV-induced abnormalities in the expression of PI3K/Akt/mTOR and its downstream regulators and alteration in host metabolism make mTOR a potential target for drug development.
-
This work was supported by a scholarship from the Medical Faculty of University Duisburg-Essen, the Foundation for Innovative Research Groups of Hubei Provincial Natural Science Foundation (2018CFA031), Hubei Province's Outstanding Medical Academic Leader Program, the National Natural Science Foundation of China (No.81974079), and the Key R&D Program of Hunan province (No.2020SK30291).
-
Open Access funding enabled and organized by Projekt DEAL.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.
-
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons. org/licenses/by/4.0/.














 DownLoad:
DownLoad: