-
Miller (37) stated, "While other viruses are studied because they cause problems, the basis of modern baculovirology was stimulated by the potential utility of baculoviruses to control insect pests." This is particularly true for viruses associated with mosquitoes as the overwhelming majority of studies have concentrated on mosquito-borne arboviruses that infect man and animals such as dengue, yellow fever and various types of encephalitis. Nysten (40) in 1808 provided perhaps the first scientific description of a baculovirus in studies on silkworms in France. However, it was more than 150 years before the first baculovirus (OcsoNPV) was isolated from the mosquito Ochlerotatus sollicitans (formerly Aedes sollicitans) in Louisiana, USA (13). Since that time, additional viruses pathogenic to mosquitoes, including cypoviruses and iridescent viruses, have been reported from many different mosquito hosts, primarily by researchers in the U.S., Europe and Russia (20). But research on these mosquito pathogenic viruses virtually came to a standstill in the 1990's because none of the viruses isolated were considered to be suitable for development as biopesticides. This was due to the inability to easily manipulate and transmit these viruses and the lack of methods for mass production. Recently, there have been tremendous advances in the ability to transmit some groups of mosquito pathogenic viruses as well as new molecular tools and capabilities to understand and manipulate these viruses at the molecular level. This review will examine the current status and recent advancements in understanding the biology and molecular features of mosquito baculoviruses, cypoviruses and iridescent viruses.
HTML
-
In the 6th report of the ICTV (39), the genera Nucleopolyhedrovirus (NPV) and Granulovirus (GV) were contained within the family Baculoviridae. Members of the genus Nucleopolyhedrovirus have occlusion bodies that contain many virions while members of the genus Granulovirus have occlusion bodies that contain one, or rarely two virions. According to an updated classification (29) four genera are now proposed for the family Baculoviridae based on host groups; Alphabaculovirus (lepidopteran-specific NPV), Betabaculovirus (lepidopteran-specific GV), Gammabaculovirus (hymenopteran-specific NPV) and Deltabaculovirus (Dipteran-specific NPV). The first report of a Deltabaculovirus (DBV) was from Ochlerotatus sollicitans (OcsoNPV) in Louisiana (13). Since then naturally occurring DBVs have been isolated from 13 mosquito species in the genera Aedes, Anopheles, Culex, Ochlerotatus, Psorophora, Uranotaenia and Wyeomyia (39) but more than 20 mosquito species have been found to be susceptible to DBV infections (4, 8, 20, 45).
DBVs are specific for midgut tissues (primarily midgut epithelium) of mainly larval mosquitoes (8, 20, 45) but infections have been found in adult midguts (9). Larvae infected with deltabaculoviruses have whitish cysts or nodules throughout the midgut and gastric caeca or isolated in one or two regions of the gut (Fig. 1A). In DBV infections the cysts are the hypertrophied nuclei of midgut epithelial cells and when the gut is examined under a phase microscope, the occlusion bodies can be seen in the nuclei (38).
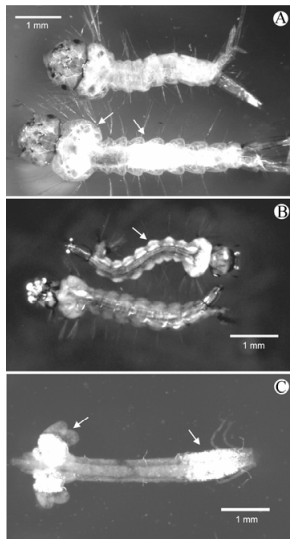
Figure 1. Gross pathology of mosquito pathogenic viruses. A: Culex quinquefasciatus larvae: healthy larva (top) and bottom larva is infected with the deltabaculovirus CuniNPV. Arrows point to regions of nuclear infection in the gastric caeca and posterior stomach. B: Ochlerotatus taeniorhynchus larvae: bottom healthy and top infected with the iridovirus R-MIV. Arrow indicates region of fat body in the larval abdominal segment infected with R-MIV. C: Dissected midgut of Culex restuans larvae infected with the cypovirus CrCPV. Arrows point to regions of cytoplasmic infection in the gastric caeca and posterior stomach.
Occlusion bodies can be relatively large and polyhedral in shape (5-20μm) or small (0.5μm) and globular in shape (8, 20, 45). The rod-shaped virions are approximately 60×250 nm and assemble in the nuclei of midgut cells (Fig. 2A). DBVs are highly virulent with infected larvae usually dying 72-96 hours post-exposure. Prior to 2001, the only detailed studies on development and transmission of DBVs were conducted with an isolate from Ochlerotatus sollicitans (OcsoNPV) from Louisiana in several different mosquito hosts (19, 22, 47) and summarized by Federici (20). Since 2001, considerable new information has been reported for a DBV from Cx. Nigripalpus (CuniNPV) (1, 8, 38) and a DBV from Uranotaenia sapphirina (UrsaNPV) (45). Information on CuniNPV will be described in detail to illustrate the current understanding of the biology, morphology and genomics of Deltabaculoviruses.
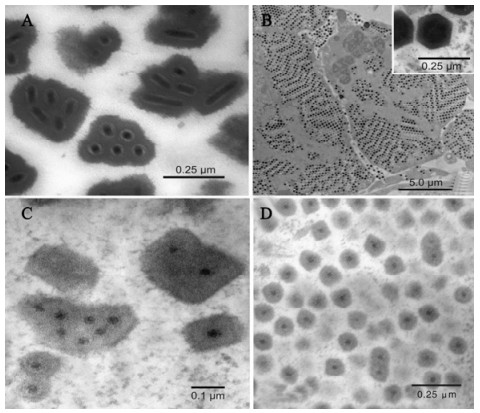
Figure 2. Electron micrographs of m+9osquito pathogenic viruses. A: Occlusion bodies of the deltabaculovirus CuniNPV in Culex quinquefasciatus. B: R-MIV in fat body cell of Ochlerotatus taeniorhynchus demonstrating virions arranged in paracry-staline arrays. The inset shows details of the icosahedral virions. C: Multiply embedded inclusion bodies of the cypovirus CrCPV in Culex restuans. D: Singly embedded inclusion bodies of the cypovirus UsCPV in Uranotaenia sapphirina.
Mature OBs of CuniNPV are uniform, globular shaped particles with a diameter of approximately 400 nm (Fig. 2A). The OBs lack an envelope (the polyhedron envelope) that is a characteristic feature of most other baculoviruses. The occlusion body protein of CuniNPv is unique among all known baculoviruses (43). Each OB typically contains 4 rod-shaped virions but sometimes contains up to 8 virions Nucleocapsids are singly enveloped and the resulting virions are approximately 200×40 nm. Each virion consists of the nucleocapsid, intermediate layer and an outer envelope. The OBs of CuniNPV do not coalesce into larger occlusion bodies as found for OcsoNPV (21) and UrsaNPV (45). Early occlusion bodies of UrsaNPV were irregularly shaped and seemed to subsequently coalesce to form large polyhedra (45). Mature occlusion bodies had a dumbbell shape, lacked the polyhedron envelope, and measured up to 10-15 μm in length and 2-3 μm in diameter.
Infections of CuniNPV are initiated when larval mosquitoes ingest occlusion bodies together with the appropriate divalent cation, usually magnesium (see below) with 2 main phases of development. Occlusion derived virions (ODVs) are released from occlusion bodies under the alkaline conditions of the midgut together with other possible factors. The released ODVs attach and pass through the peritrophic matrix (PM) followed by attachment to the membrane of the microvilli and entry into the cytoplasm of midgut epithelial cells. Nucleocapsids attach to the nuclear envelop where DNA is released into the nucleoplasm to initiate viral replication. This process of ingestion of infectious particles to the invasion of the nucleus is a rapid process and occurs within 2-4 hours post exposure to occlusion bodies. The next phase of the process involves the rapid spread of CuniNPV to other midgut cells and is believed to involve budded virions (BV). The initial viral replication produces nucleocapsids that are released from the nucleus that form extracellular virions (the BV) which are released from the midgut cells into the ectoperitrophic space (38). The BV's then attach to microvilli of other midgut cells and make their way to the nucleus to initiate another round of replication. At some point, nucleocapsids acquire de novo envelops in the nucleus and become occluded by the occlusion body protein. The nuclei become packed with occlusion bodies which are released upon death of the host and cause infection when ingested by a susceptible mosquito larva. The spread within the mosquito midgut is rapid with production of occlusion bodies within 14-48 hours post exposure.
Prior to work with CuniNPV, transmission studies had only been conducted with the NPV from Oc. sollicitans (OcsoNPV). Transmission tests with Ocso NPV to the natural host generally yielded low infection levels that averaged only about 14% (14). While infection levels for OcsoNPv did vary with the host and age of mosquitoes tested, the virus was generally difficult to consistently transmit (48). The initial attempts to transmit CuniNPV to larval mosquitoes in the laboratory were also disappointing and usually resulted in less than 1% infection levels (8). This changed dramatically when divalent cations were determined to play a crucial role in the transmission of CuniNPV. Deletion analysis of the principle cations present in the field water was used to determine the waterborne factors critical for transmission of CuniNPV. Bioassays of Cx. quinquefasciatus larvae exposed to Cuni NPV were conducted in a salt mixture based on analysis of the field water. The addition of salts to deionized water significantly improved the infection levels in larvae. Salt mixtures without Mg2+ resulted in less than 1.0% infections. Salts with Mg2+or without Ca2+ increased infection levels (8). Further investigations revealed conclusively that transmission is mediated by divalent cations: magnesium is essential, whereas the presence of calcium inhibits the activity of magnesium to mediate transmission. Transmission of UrsaNPV is also driven by cations similar to that found for CuniNPV (45). Transmission of CuniNPV can also be enhanced by the divalent cations barium, cobalt, nickel, and strontium or inhibited by copper, iron, and zinc (4, 8).
To produce high levels of infection in mosquitoes with CuniNPV, Mg2+ must be present only during the first hours of CuniNPV exposure indicating that the activity of Mg2+ occurs early in the infectivity process. Observations of occlusion bodies in the midgut lumen revealed that Mg2+ is probably not required for dissolution (38). Possible targets requiring Mg2+ are proteases that specifically attack the PM allowing virions to cross this barrier to infection or to facilitate the attachment of virions to receptors on the PM or microvilli of midgut epithelium to facilitate entry into the cells.
CuniNPV has only been transmitted to mosquitoes within the genus Culex, subgenus Culex (4, 8). These include Cx. nigripalpus, Cx. quinquefasciatus, Cx. salinarius, Cx. pipiens, Cx. pipiens f. molestus and Cx. restuans. Cx. territans (subgenus Neoculex) was the only Culex species tested that was not susceptible to CuniNPV (4). No infections were found with any species of Aedes (Ae. aegypti, Ae. albopictus), Anopheles (An. albimanus, An. quadrimaculatus), Ochlerotatus (Oc. triseriatus, Oc. taeniorhynchus), Culiseta melanura or Toxorhynchites ambionensis. The very restricted host range of CuniNPV to only Culex spp. differs from previous transmission studies with OcsoNPV that found Aedes, Ochlerotatus and Psorophora spp. susceptible but not Culex and Anopheles spp. (14, 48).
The complete genome of CuniNPV has been sequenced and found to be a circular dsDNA molecule of 108 252 base pairs (50.9% GC composition), which encodes 109 putative genes (1, 38). There are 36 genes in the CuniNPV genome with homologues to other baculoviruses, however its lack of conservation in gene order, and the absence of homologues of numerous genes present in known lepidopteran and hymenopteran baculoviruses, suggest significant evolutionary distance between Dipteran and other baculoviruses (1, 24, 31). Phylogenetic analysis of 13 baculoviruses (27) separated the group into four distinct lineages; Group 1 NPVs, Group 2 NPVs, Granulosis viruses and CuniNPV. The authors concluded that CuniNPV represents a new baculovirus genus supporting the earlier conclusions of Moser et al. (38) and has resulted in the creation of the genus Deltabaculovirus for mosquito baculoviruses (29).
CuniNPV contains genes with potential host range functions including a p35 homologue (Cun75, antiapotosis gene), homologues of per os infectivity factors (pif-1, Cun29, pif-2, Cun38 and pif-3, Cun46) that are important in infectivity of lepidopteran and hymenopteran baculoviruses (30, 31, 41, 44). The presence of novel cellular homologues suggests unique mechanisms of viral-host interaction in mosquito baculoviruses and might have application for the identification of novel genes and gene products.
-
There are currently five genera of iridoviruses (Ⅳ) represented by those found in vertebrate hosts (Ranavirus, Lymphocystivirus, and Megalocytivirus) and those from invertebrates (Iridovirus and Chloriridovirus). Mosquito iridescent viruses (MIV) are members of the genus Chloriridovirus (50, 51) and currently contains only the type IIV3 from Ochlerotatus taeniorhynchus (6, 15). It is currently recognized that MIVs are distinct from all known iridescent viruses and that the diversity among invertebrate iridescent viruses may be greater than previously recognized (17)
The MIVs are large, icosahedral viruses (approximately 180 nm in diameter) that replicate and assemble in the cytoplasm (Fig. 2B). The genome is composed of a single molecule of linear doublestranded DNA (17) MIVs have been reported in the US, Russia and Europe from Aedes, Psorophora, Culex and Culiseta spp. Infections in larvae are usually easily detected due to the iridescent color produced by the paracrystalline arrays of virions in fat body (Fig. 2B) and vary from yellow-green or orange and sometime turquoise (Fig. 1B). The originally isolated virus in Oc. taeniorhynchus was designated as RMIV (regular mosquito iridovirus) that produces an orange iridescence in larvae (36). This was to separate it from a spontaneously formed laboratory strain of the virus designated TMIV (turquoise mosquito iridovirus) that produces a bluegreen iridescence in Oc. taeniorhynchus.
The virions of MIVs are icoshedral with an electron dense core of DNA surrounded by an intermediate membrane-like lipid layer contained within a proteinaceous capsid (Fig 2B, inset). Virions range is size from 180-200 nm in fixed sections. The principle site of infection for MIVs is larval fat body with the epidermis as the next most commonly infected tissue.
RMIV is host specific, infecting only Oc. Taeniorhynchus and Oc. sollicitans (7, 52). The virus is horizontally transmitted and usually results in infection levels of less that 20% (49, 52). RMIV can also be transmitted transovarially with high levels of infection in progeny (23, 26, 32, 52). Infected larvae usually die as fourth instars due to destruction of the fat body. The natural transmission cycle of RMIV in Oc. taeniorhynchus populations has been proposed to occur by alternating horizontal and vertical transmission events (32).
To evaluate the non-susceptibility of most of the mosquito population exposed to RMIV, Undeen and Fukuda (49) examined the possibility that resistance was genetic and that RMIV has no specific means of entering the host. To test for genetic resistance, mean infection rates in randomly selected and sibling Oc. taeniorhynchus larvae were compared. There was no difference in infection levels between the two groups rendering genetic resistance unlikely. Injury to larvae by feeding silicon carbon fibers consistently yielded higher infection rates (17%) compared with only 4% infection rates for virus alone. These authors concluded that RMIV has no active means of entering the larval host but invades through breaks in the cuticle or peritrophic matrix.
Mosquitoes are also susceptible to invertebrate iridescent virus 6 (IIV-6) which was originally isolated from Chilo suppressalis (Lepidoptera: Pyralidae) which is the type for the genus Iridovirus (51). Sublethal (covert) infections in adult Ae. aegypti resulted in reduced longevity, smaller body size and reduced fecundity than non-infected conspecifics (33, 34, 35). The net reproductive rate for covertly infected females was reduced by 50% (34).
With the recently published DNA sequence and analysis of the Invertebrate iridescent virus type 3 (IIV-3) for the Chloridovirus, the complete genomes have been determined for viruses representing all Ⅳ genera (17). RMIV has a 190, 132 bp linear genome with a 48% GC content. There are 126 predicted IIV-3 genes of which 27 are present among all currently sequenced Ⅳs suggesting a genetic core for the family Iridoviridae. Analysis revealed that IIV-3 most closely resembled IIV-6 (Chilo iridescent virus, type species of the genus Iridovirus) and shared fifty-two ORFs which were not present in the vertebrate Ⅳ counterparts. This may reflect distinct evolutionary histories for vertebrate and invertebrate Ⅳs and suggests a role for these genes in aspects of virusinvertebrate host interactions. It was concluded that IIV-3 contained a number of novel genomic features indicating that it is distantly related to other Ⅳ genera and confirmed its unique position within the Iridoviridae (17).
-
The genus Cypovirus within the Reoviridae contains the insect cytoplasmic polyhedrosis viruses (CPVs) (10). CPVs have been isolated from more that 250 insect species collected both in the laboratory and from the field with the great majority of these from lepidopteran hosts (28). Numerous viruses from the larvae of 20 different species of mosquito (repressenting 9 different genera), have been identified as cytoplasmic polyhedrosis viruses based on similarities to viruses from Lepidoptera (20). These similarities included virion structure, the presence of inclusion bodies (polyhedra) and chronic infection in the cytoplasm of larval midgut epithelial cells. There were also three reports of CPVs from the midgut epithelium of adult mosquitoes (11, 16, 18). Since Federici (20) summarized the status of CPVs, nearly 20 years have passed without any appreciable new knowledge on this common group of viruses in mosquitoes and their taxonomic status has been in limbo. However, this has recently changed with new studies on the biology, morphology and molecular characteristics of CPVs from Uranotaenia sapphirina (UsCPV) and Culex restuans (CrCPV) establishing these as members of the new Cypovirus-17 species group (25, 46).
Larvae infected with Mosquito Cypoviruses (MCPVs) have whitish cysts or nodules in the midgut. These can be throughout the midgut and gastric caeca or isolated in one or two regions of the gut (Fig. 1C). The inclusion bodies of cypoviruses are restricted to the cytoplasm which appears blue to white and the nuclei are compressed, almost not detectable. The infected cells will look irregular; they usually do not disintegrate in water and retain their cell membrane when smeared in water.
MCPVs are characterized by isometric virions approximately 60 nm in diameter (25, 46). Occlusion bodies are of two types: small cuboidal inclusion bodies that contain only one (Fig. 2D) or a few virions (5, 46) and large irregularly shaped inclusion bodies (Fig. 2c) that contain many virions (2, 3, 13, 14, 18, 25). MCPV development is restricted to the cytoplasm of cells of the gastric caeca and posterior stomach (2, 3, 5, 18, 25, 46). The first report of a MCPV was from Cx. salinarius collected in Louisiana (12). Currently, there are reports of MCPVs from about 20 mosquito species representing 7 genera. Recent new information for MCPVs since these earlier reports is for UsCPV (46) and CrCPV (25) and summarized below.
Patent infections of MCPVs produce a characteristic porcelain-white or iridescent color in midgut cells as a result of the accumulation of occlusion bodies in the cytoplasm (25, 46). MCPV infections are localized to the gastric caeca and the posterior portion of the stomach (20). A novel cypovirus from Uranotaenia sapphirina (UsCPV) is characterized by the production of large numbers of virions and inclusion bodies, and their arrangement into paracrystalline arrays gives the gut of infected insects a distinctive blue iridescence (46). MCPVs are usually benign with most infected individuals surviving to the adult stage (20, 25, 46).
Cypovirus virus particle structure differs from vertebrate, plant and other insect reoviruses by the absence of outer capsid layers and by the production of a viral coded polyhedron protein that occludes the virions (10). The virions of UsCPV, CrCPV and most other MCPVs are icosahedral (55 to 65 nm in diameter) with a central core that is surrounded by a single capsid layer. In general the development of UsCPV and CrCPV appears to be similar to that of other mosquito cypoviruses (2, 3, 20). These viruses are assembled within virogenic stroma and occluded singly (occasionally multiply) by the deposition of a crystalline polyhedron matrix around individual particles. Although a similar process for formation of inclusion bodies has been reported in An. Quadricmaculatus (5), it is different from that observed in other mosquitoes. In CrCPV (3, 25) polyhedra are also formed by deposition of polyhedron around individual particles, although these subsequently coalesce to form larger pleomorphic inclusion bodies. In Oc. cantator (2) and Oc. taeniorhynchus (18) relatively large inclusion bodies are formed in clusters by deposition of protein around groups of virions. Thus, the majority of inclusion bodies in these mosquitoes are large, irregularly shaped and contain multiple virions. In contrast, the great majority of UsCPV polyhedra [and the virus reported in An. Quadricmaculatus by Anthony et al., (5)] contain only one virion and are cuboidal in shape.
UsCPV has a relatively broad host range including Cx. quinquefasciatus, Ae. aegypti, Oc. triseriatus, Ur. lowii, and An. albimanus, although it was not transmitted to An. quadrimaculatus or the lepidopteran host H. zea. Previous per os transmission studies with mosquito CPVs were successful and also exhibited a certain degree of specificity (3, 13, 14). This indicates that there may be a number of distinct mosquito CPVs several of which can infect the same mosquito host species. Cx. erraticus and An. crucians may also act as hosts for UsCPV since they were found together with infected Ur. sapphirina in the field and exhibited similar infection characteristics including the iridescent color (46).
Horizontal transmission of several MCPVs has been demonstrated through direct exposure of mosquito larvae to inclusion bodies but infection levels were generally low (2, 3, 14, 18). Recent studies by Shapiro et al., (46) and Green et al., (25) have shown that oral transmission of UsCPV to mosquito larvae is enhanced by magnesium and inhibited by calcium ions. UsCPV exposed to larvae in deionized water was non-infectious for Ae. aegypti larvae but the infection rate was significantly increased by the addition of 10 mmol/L Mg2+ ions (46). This was also the case in transmission experiments with CrCPV in Cx. Quinquefasciatus (25). However, the addition of 10 mmol/L Ca2+ ions to this mixture inhibited infection in both UsCPV and CrCPV. Most of the UsCPV and CrCPV infected larvae survived to adulthood and retained the infections in the midgut (25, 46). This indicates that UsCPV may be vertically transmitted to the filial generation similar to what has been reported for an MCPV from Oc. sollicitans (14) and CrCPV (3).
Cypoviruses usually have a ten segmented dsRNA genome and the different 'types' are initially identified on the basis of differences in the migration patterns of their genome segments during polyacrylamide gel electrophoresis (42). Analysis of the UsCPV and CrCPV genomes by 1% AGE generated ten RNA bands (25, 46). Electrophoretic analysis of the UsCPV and CrCPV genome segments demonstrated a similar migration pattern (electropherotype) but different from those of the sixteen Cypovirus species already recognized. The smallest band (segment 10) encodes the viral poyhedrin protein. The UsCPV and CrCPV polyhedron sequences are similar but showed no significant nucleotide similarity to the corres ponding segment of the other cypovi-ruses that have previously been analyzed and it has different 'cons-erved' termini (25, 46). It was concluded that UsCPV and CrCPV should be recognized as a member of a new Cypovirus species (Cypovirus 17: Strain UsCPV-17 and CrCPV-17).
-
Genomic information on mosquito pathogenic viruses and their mosquito hosts is rapidly accumulating and offers exciting possibilities to understand virus-mosquito interactions at the molecular level. These studies can address basic and important properties of these viruses such as transmission, host range, safety, virulence and other biological features. Molecular studies should also provide fundamental taxonomic characteristics as well as the possibility of modifying these viruses to use as tools for laboratory studies or to enhance traits to make them more viable as biocontrol agents. There is still a great need to isolate new viruses (or previously described ones) from various geographical locations to build a repertoire of viruses and characterize their unique biological and molecular properties. Understanding how these pathogens exploit their host provides the opportunity to devise novel research and control strategies for the development of mosquito pathogenic viruses as components of integrated mosquito control programs.













 DownLoad:
DownLoad: