-
Herpes simplex virus type 1 (HSV-1) is a DNA virus with rapid proliferation and high infectivity. It is a common human pathogen that occurs worldwide with 60% to 95% of infection in adults, and is responsible for a broad range of human infectious diseases such as gingiva-stomatitis, keratitis, cutaneous herpes, genital herpes and encephalitis [17, 18].
Herpes simplex virus infections are usually managed with anti-viral drugs such as acyclovir, vidarabine and phosphonoformic acid [12]. However, undesirable complications and the emergence of drug-resistant virus strains necessitate the development of alternative agents for the management of HSV infections [7, 11].
Chinese traditional knowledge and practices play an important role in the discovery and development of safe and effective drugs. Natural products remain a consistently successful source in drug discovery and may offer many opportunities to find anti-HSV drugs or lead compounds [5, 20]. A variety of natural products have been found effective on inhibiting unique enzymes and proteins crucial to the virus life cycle [2, 21].
The plant Tripterygium hypoglaucum (level) Hutch (Celastraceae) is distributed along the South bank of the Yangtze River and in Southwest regions of China. It has been used as a traditional medicine with the pharmacological activities of anti-inflammation, anti-tumor, antifertility and immunosuppression, and employed for the treatment of rheumatoid arthritis, acute infectious hepatitis, chronic nephritis, leukaemia, erythematous lupus, and neurodermatitis [4].
The medicinal part of T. hypoglaucum is its roots with principal active components of alkaloids, terpenoids and tannins [10]. The content of total alkaloids in the root is about 1% which is obviously higher than in the stem. The compounds purified from root methanol extracts, such as triptonine A, hypoglaunine A, hypoglaunine B and hyponine E, were reported to be active in anti-HIV virus [8]. A primary investigation was carried out on the treatment of viral keratitis with the preparation of T. hypoglaucum.
In this paper, the in vitro anti-HSV-1 activity of the total alkaloids extract from T. hypoglaucum was evaluated at the cellular and molecular level.
HTML
-
Reagents for cell culture, such as DMEM medium, fetal bovine serum (FBS), EDTA and HEPES were purchased from GIBCO. Dimethyl sulfoxide (DMSO), 3-(4, 5-dimethyl thiazole-2)-2, 5-diphenyl thiazolyl blue (MTT) were purchased from Sigma. Molecular reagents such as Trizol, M-MuLV reverse transcriptase, dNTP mixture, Oligo dT, PCR Master Mix, DL2000 DNA marker were purchased from TaKaRa. PCR primers were synthesized by the Shanghai Invitrogen Biotech Corporation.
-
The medicinal plant T. hypoglaucum (level) Hutch was collected in Yunnan province, China. A voucher specimen (IBSC-398766) was authenticated by Prof. Cheng Huang and deposited in the herbarium of the South China Institute of Botany, Chinese Academy of Sciences.
-
The harvested roots of T. hypoglaucum were washed, dried and powdered. A thousand grams of powdered material was extracted with 85% ethanol by maceration for 1 h and under hot reflux for 3×1 h, solid extract was obtained by evaporating solvent from the filtered extracts under reduced pressure, dissolving with water, then drying by lyophilization. Twenty grams of the concrete powder was dissolved in 80 mL of 1% hydrochloric acid and extracted at 80℃ for 1 h, adjusting the pH value of the filtered solution to 9.0 with aqueous ammonia, extracted with ethyl acetate again. The total alkaloids extract was achieved after evaporating the solvent.
Before testing, the alkaloids extract was dissolved in DMSO, diluted with maintenance DMEM medium and filtered with 0.22 μm membrane to form 42.7 mg/mL of stock solution.
-
Vero cells (African green monkey kidney cell, ATCC: CCL-81) were grown under 5% CO2 at 37℃ in DMEM medium supplemented with 10% (v/v) FBS and 2% for cell maintenance. A virus stock of HSV-1 (standard strain Sm44, provided by the Institute of Virology, Wuhan University, China) were routinely prepared from infected Vero cells and stored in small aliquots at −80℃. The 50% tissue culture infective dose (TCID50) was determined by cytopathic effect (CPE) method and calculated by Reed-Muench method [15]. Titer of the virus stock was 105.5 TCID50 /mL.
-
The cytotoxicity of the total alkaloids extract on Vero cells was evaluated before antiviral activity assay by MTT method as described elsewhere [13]. Briefly, cells (2×105/mL) were seeded in a 96-well tissue culture plate and incubated at 37℃ in 5% CO2 for 24 h to form cell monolayers. After gently removing the medium in each well, 2-fold serial extract dilutions in maintenance medium were added to cell monolayers successively in triplicate (wells without extract were treated as control). After 48 h of incubation at 37℃, cytotoxicity was determined by microscopic examination of cell morphology compared with the control. The supernatants were discarded and MTT reagent was added to each well and incubated for 4 h, 100 μL of DMSO was added and incubated 1 h. The absorbance values were read at 570 nm (A570) on a Bio-Tek ELISA reader. The 50% cytotoxic concentration (CC50) and cell viability were calculated from the dose-response curve. Cell viability (%) was calculated as (A570 of the treated / A570 of the control) × 100.
-
Antiviral activity of the total alkaloids extract was determined according to the inhibitory capacity against the viral cytopathic effect [24]. HSV-1 virus stock was diluted into 100 TCID50 with maintenance medium. The extract stock solution was 2-fold serially diluted into seven test concentrations subjected to the MTC, then mixed with an equal volume of virus dilution. The mixtures were inoculated onto cell monolayers in 96-well plate (100 μL/well, 3 parallel wells) and incubated at 37℃ for 72 h. Virus control, cell control, and positive control (Acyclovir, Sigma) were set simultaneously. CPEs in all test groups were recorded while wells of the virus control measured 100% CPE. The 50% inhibitory concentration (IC50) was calculated from the Reed-Muench formula. The therapeutic index (TI) was calculated using the formula: TI=CC50/IC50
-
Vero cells (2×105/mL) were seeded in a 96-well plate and incubated in 5% CO2 at 37℃ for 24 h to form confluent monolayers. The medium was discarded, then cells were inoculated with 100 μL of highly diluted HSV-1 virus supplemented with 100 μL of the total alkaloid extract (positive control, virus control and cell control were also set), and left to adsorb for 1 h at room temperature on a tray shaker. After adsorption, the inoculate was replaced with 1.0 mL of overlay medium (DMEM medium with 1% carboxymethyl cellulose) and incubated in 5% CO2 at 37℃ for 72 h. After discarding the overlay medium, the cells were fixed by adding 1 mL of 5% formalin for 5 min, washed with running tap water, followed by staining with 0.8% crystal violet in 50% ethanol. The viral plaques were counted under a dissecting microscope. Overlapping plaques and plaques at the edge of the well were counted as a single plaque.
-
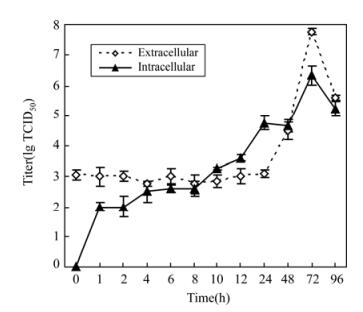
Determination of virus multiplication curve. A virus multiplication curve was calculated to determine the optimum sampling time for virus RNA extraction. Vero cells (2×105/mL) were seeded in a 96-well plate and incubated in 5% CO2 at 37℃. When confluent monolayers formed, the medium was discarded, then inoculated with 100 μL of HSV-1 virus dilution (100 TCID50). At 1, 2, 4, 6, 8, 10, 12, 24, 48, 72, 96 h after infection, the infected cells and supernatant were collected separately, and intracellular and extracellular virus titers were determined by CPE method. Then, the proliferation curve of virus titer versus sampling time was obtained.
Cell treatment and RNA extraction. Vero cells were seeded in a 6-well plate (2×105/mL, 3 mL/well) and incubated into confluent monolayers. The medium was removed, inoculated with HSV-1 virus dilution (100 TCID50) and incubated at 37℃ for 2 h, then the virus supernatant was replaced with alkaloid extract solution (12.5 μg/mL) and further cultured in 5% CO2 at 37℃. Total RNAs of the harvested cells (3 groups: test group, positive control, virus control) were extracted using the Trizol method, and their purity and concentration were determined by analyzing the absorption value at 260/280 nm with a Nucleic acid-Protein Analyzer (BECKMAN).
RT-PCR analysis. The first cDNA chains were synthesized from the extracted mRNA templates by reverse transcription, then used as templates for the amplification of virus genes US6, UL30, UL39 and a celluar inner control gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) by polymerase chain reaction (PCR). Primers for PCR are listed in Table 1.

Table 1. The primes for gene amplification
PCR conditions were as follows (20 μL): PCR Master Mix 10 μL, primes 2μL, cDNA templates 3 μL, 3d H2O 5 μL. The amplification conditions were: 94℃ 5 min; 94℃ 40 s, 58℃ 40 s, 72℃ 40 s, for 28 cycles; 72℃ 8 min; 4℃ forever.
When PCR reaction was complete, 6 μL of the products were identified by electrophoresis with 1% agarose gel, then the gel profiles were scanned and analyzed with the Bioimaging System (SYNGENE). The ratio value of the integrated optical density of aimed gene to that of GAPDH was assessed as the indication of gene expression level.
Reagents
Plant material
Preparation of the total alkaloids extract
Cell and virus
Cytotoxicity assay
Antiviral activity by CPE assay
Plaque inhibition assay
Inhibitory activity of the extract against the expression of gene US6, UL30 and UL39
-
Cytotoxicity of the crude alkaloids extract against Vero cells was measured in order to gauge antiviral selectivity by MTT assay after a 48 h of co-incubation with drug dilutions. The cytotoxic concentration that caused the reduction of viable cells by 50% (CC50) for total alkaloids and Acyclovir was 46.6 μg/mL and 841.3 μg/mL respectively (Table 2).

Table 2. Cytotoxicity of the total alkaloids extract to Vero cells
-
The inhibition activity of the alkaloids extract from T. hypoglaucum against HSV-1 induced CPE was investigated and results are shown in Table 3. The results indicate that the alkaloids extract possessed a potent inhibitory activity against HSV-1 with an IC50 value of 6.5 μg/mL which was notably lower than that of Acyclovir (15.4 μg /mL).

Table 3. Antiviral activity against HSV-1 by CPE assay
-
The improved Antiviral activity of the alkaloids extract compared to Acyclovir was further confirmed by plaque inhibition assay. The results (Table 4) show that the extract significantly reduced the viral plaque formation at a low concentration of 6.25 μg/mL to 12.5 μg/mL with the plaque reduction ratio of 55% to 75% which was 35% higher than that of Acyclovir at the same concentration, and the inhibition activity showed an obvious dosage dependence.

Table 4. Plaque reduction activity against HSV-1
-
Before investigating the inhibition effect of the extract on gene expression, a growth curve of HSV-1 virus proliferation was determined (Fig. 1). Intra-cellular virus titers increased continually from 1 h to 24 h after inoculation, but extracellular virus titers were constant at this stage, subsequently, both titers increased and reached peak values at 72 h. Based on this result, cells were harvested at 24 h after virus infection and gene expression levels were measured.
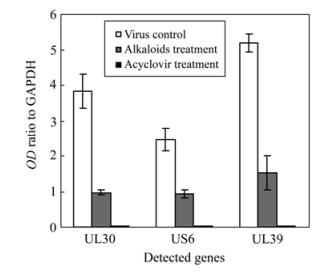
Three characteristic bands of the total RNA extracts were clearly visible on agarose gel after electrophoresis. RT-PCR results of electrophoresis gel profiles and the integrated optical density ratio of cDNA bands are shown in Fig. 2 and Fig. 3. At the same concentration of 12.5 μg/mL, Acyclovir showed almost complete expression inhibition to all the 3 detected virus genes. The alkaloids extract also showed high inhibition activity against the expression of the 3 genes at 12.5 μg/mL, with an inhibition efficacy of 74.6% (UL30), 70.9% (UL39) and 62.6% (US6) respectively compared to the virus control.
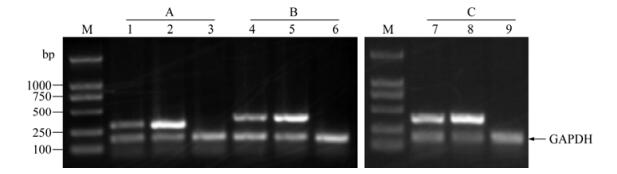
Figure 2. RT-PCR detection of virus gene UL30, US6, UL39 and inner control GAPDH. Group A (lane 1-3) for UL30 gene detection; Group B (lane 4-6) for US6 gene; Group C (lane 7-9) for UL39 gene. Lane 1, 4 and 7, Samples from virus infection + alkaloids extract treatment; Lane 2, 5 and 8, Virus control (no drug treatment); Lane 3, 6 and 9, Positive control (Acyclovir treated); M, DL2000 DNA marker.
Cytotoxicity of the total alkaloids extract
Inhibition activity of the total alkaloids extract against HSV-1 viral CPE
Plaque reduction effect of the extract
Inhibition effect of the extract on gene expression
-
Botanical extracts exhibit a wide spectrum of biological and pharmacological properties, including cytoprotection, anticancer, anti-inflammation, immuno-modulation and anti-infection [14, 22]. Such medicinal plants provide an important source for antiviral drug screening and development [6, 1, 9].
The components of T. hypoglaucum are complex, but the main medicinal content in the root has been identified to comprise alkaloids. In this study, we found that cytotoxicity of the crude alkaloid extract was larger than for Acyclovir (ACV), with a TI of 7.17 compared to 54.63 for ACV (Table 3). This showed the drug safety of the crude alkaloids extract was less than ACV's. But the crude alkaloids extract displayed significant activity against HSV-1 and inhibited the proliferation of HSV-1 in Vero cells. Its IC50 was lower than that of Acyclovir (Table 3), and presented notably higher plaque reduction efficacy than Acyclovir at a concentration range of 6.25 to 12.5 μg/mL (Table 4). RT-PCR assay further suggested the potent anti-HSV-1 activity.
Theoretically, antiviral activity could take place at many different steps of the viral lifecycle, such as host invasion, genome transcription, DNA replication, particle assembly, virion maturation and expression. Thus, through modern biological and chemical assays, the action mechanism may be well revealed.
HSV-1 is an enveloped virus with a large double-stranded DNA molecule. The genome is about 152 kbp and usually be divided into L-region, S-region (each owns unique sequences, marked as UL and US) and L-S junction. To date, about 80 genes have been identified, 56 genes are located within the UL region and 12 genes in the US region. All HSV-1 genes are transcribed by the DNA dependent RNA transcriptase of host cell and fall into three main transcription regulation classes: immediate early (IE), delayed early (DE) and late (L) genes [19]. UL30 is a type of IE gene that codes virus DNA polymerase, UL39 is also an IE gene encoding a subunit of virus ribonucleotide reductase, they play important role in virus genome replication. US6 is a L gene encoding glycoprotein gD which is the main component of virus envelope and acts later role in entry.
The functions of about half the HSV-1 coded proteins have been shown to be absolutely necessary for successful lifecycle [16]. Any one of these indispensable virus proteins identifies a possible target for antiviral intervention. The most successful antiviral directed against HSV to date is Acyclovir. Acyclovir interacts specifically and sequentially with the proteins coded by UL23 and UL30. Acyclovir is phosphorylated to its active form by the viral encoded thymidine kinase where it acts as a chain terminator of the viral DNA polymerase there by stopping any further viral replication [3, 23].
In the present study, transcription levels of the 3 key genes UL30, UL39 and US6 were semi-quantified by RT-PCR assay (Fig. 2, Fig. 3). Under treatment with T. hypoglaucum alkaloids extract at a concentration of 12.5 μg/mL, the transcription of genes UL30, UL39 and US6 was significantly inhibited by an inhibition efficacy of 74.6% (UL30), 70.9% (UL39) and 62.6% (US6) respectively contrasted to virus control, which further confirmed the potent antiviral activity against HSV-1 replication though lower than that of Acyclovir.
In conclusion, the total alkaloids extract of the medicinal plant T. hypoglaucum presented potent anti-HSV-1 activity and low cytotoxicity in Vero cells. It is worthwhile to do further works on the identification of potential active compounds and the investigation of action mechanism.













 DownLoad:
DownLoad: