-
The family Retroviridae is divided into two different subfamilies, Spumaretrovirinae and Orthore-trovirinae . Foamy viruses (FVs) are the only genus of the Spumaretrovirinae subfamily. Besides a variety of primate species such as chimpanzees and baboons, FVs have also been isolated from other hosts including bovine, equine and feline species [1, 4, 5, 9, 19]. Distinct from Orthoretrovirinae , such as human immunodeficiency virus and human T-cell leukemia virus, viruses of this subfamily have two promoters in their genomes. The 5' long terminal repeat (LTR) promoter directs the expression of the structure genes, gag, pol and env , and the internal promoter (IP), which locates towards the 3' end of the env gene, regulates the expression of the accessory proteins, Tas and Bet.
Interaction and subsequent self-multimerization of Gag protein cause capsid formation [3, 18]. Unlike other retroviruses, which produce Gag-Pol fusion proteins, the Gag and Pol proteins of FVs are produced from separate RNA transcripts [21]. All data in FVs indicate that the biological significance of the specificity of the proteolytic processing events of Gag play a prominent role in viral maturation and assembly. For example, there are four processed forms of Gag protein, which is divided into two kinds: optimal cleavage, p68/p3 and suboptimal cleavage, p33/p39 or p39/p29 [13, 14]. Several regions were identified on the human foamy virus (HFV) Gag protein to serve corresponding functions: Three glycine (G)/arginine (R)-rich sequences (GR boxes) in the C-terminus are implicated in viral nucleic acid binding and harbor a nuclear localization sequence (NLS) [15, 22], a 18 amino acids motif (amino acids 43-60) resembling the cytoplasmic targeting and retention signal (CTRS) of type D retroviruses allows cytoplasmic targeting of Gag [3]; and a coiled-coil domain (amino acids 130-160) is necessary for Gag-Gag interaction [18]. The last two motifs are required for capsid assembly. Viral genome targets and concentrates Gag protein to initiate Gag-Gag interaction, and then this interaction leads to particle transport into the plasma or intracellular membranes and ultimately to egress form the host cells [8]. Up to now, compared to studies of the viral minus-ends transportation to the cellular nucleus, few studies focus on how the assembling viral particles transport into cellular membrane.
Microtubules (MTs) are long, hollow cylinders assembled from equally oriented heterodimers of α-and β-tubulin and MT-associated proteins [11]. Polar MTs with distinct minus-and plus-ends are the highways for long distance transport; their ATP-hydrolyzing motors are dyneins and kinesins. Cytoplasmic dynein, which comprises a 20 S protein complex consisting of two dynein heavy chains (DHCs; 520 kDa), two dynein intermediate chains (DICs; 74 kDa), several dynein light intermediate chains (DLICs; 53-57 kDa) and a series of dynein light chains (DLCs) from the LC8, Tctex/rp3 and LC7/roadblock families, catalyzes minus-end-directed microtubule (MT) trans-port [7]. Viruses are obligate intracellular parasites and therefore depend on the cellular machinery for cellular trafficking [2]. For example, the interaction between Gag and LC8 probably accounts for intracellular minus-ends transport of HFV along the MTs [12].
In this study, we cloned the BFV gag gene into prokaryotic expression vector pET 28a and purified denaturalized Gag protein. The protein was used to immunize BALB/c mouse to produce antiserum, which could recognize specifically the BFV Gag protein in BFV-infected cells through western blot assay. Additionally, these results demonstrated that both the optimal and suboptimal cleavages of Gag protein occur in BFV-infected cells. Subsequently, the Gag antiserum was used to investigate subcellular localization of BFV. In immunofluorescence micros-copy assay, colocalization microtubules (MTs) and assembling viral particles was clearly observed, which implied that BFV may transport along cellular MTs in host cells. Furthermore, MTs-depolymerizing assay indicated MTs were required for the efficient replication of BFV. In conclusion, our study suggests that BFV has evolved the mechanism to hijack the cellular cytoskeleton for its replication.
HTML
-
The complete Gag cDNA was amplified by PCR from BFV genome DNA by using the following primers, Forward: 5'-aacgaattcaccgtctcag-3', Reverse: 5'-cagctcgagttaagatgattg-3' and inserted into pET28a which has an N-terminal His-tag and pcDNA 3.1+ for expression in eukaryotic cells.
-
The prokaryotic expression plasmid pET28a-Gag was transformed into competent E. coli BL21 (DE3) (Invitrogen) and positive colonies were selected by ampicillin antibiotics. In short, the desired clone grew at 37 ℃ overnight, was inoculated into fresh media and grew for an additional 3 h (A600=0.6-0.8) and then was induced at 37 ℃ overnight by adding isopropyl β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mmol/L to the E. coli culture. After overnight culture, cells were harvested in PBS, sonicated, and centrifuged at 4 ℃ (10 000 ×g, 15 min). The deposit was washed with PBS (additional 2 mol/L NaCl and 1 mol/L urea) 3 times, and dissolved with denaturalization buffer (6 mol/L urea).
-
Fetal bovine lung (FBL) and BFV indicator cell line (BFVL) cells, harbouring the open reading frame of Luciferase driven by BFV long terminal repeat (LTR) (Hong-yan Guo, unpublished data), were maintained in DMEM (Dulbecco's Modified Eagle's Medium) supplemented (Gibco-BRL) with 10% fetal bovine serum (Hyclone), streptomycin (50 μg/mL) and penicillin (50 U/mL) as monolayers in tissue culture plates. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37 ℃. BFV (3026 strain) was isolated from peripheral lymphoid cells by us in 1993. BFV was propagated in FBL cell.
-
Cells were lysed and proteins were separated by 12% SDS-PAGE (polyacrylamide gel electrophoresis), and the gels were subsequently blotted onto a polyvi-nylidene difluoride membrane (Millipore Billerica, MA) by electroblotting for 1 h at 100 V at 4 ℃. Then the membrane was blocked in 5% non-fat milk-PBS (phosphate-buffered saline) for 45 min at room temperature, and then incubated with primary antibody for 90 min at room temperature followed by goat anti-mouse Ig peroxidase-conjugated secondary antibodies purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
-
Cells grown on glass coverslips in 12-well plates were fixed with 4% (w/v) paraformaldehyde-PBS for 10 min and then permeabilized in 0.5% Triton X-100-PBS for 10 min. Cells were blocked by incubating with 1 mL of 2% bovine serum albumin (BSA) in PBS at 37 ℃ for 30 min. Antibodies against Gag and α-tublin (Cytoskeleton Company) were diluted 1:500 and incubated with the coverslips at 37 ℃ for 1 h. Cells were then washed with 2% BSA/PBS for 10 min at room temperature before incubating with a 1:1 000 dilution of Texas Red-conjugated goat anti-mouse and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibodies (purchased from Jackson ImmunoResearch Laboratories) at 37 ℃ for 30 min. Nuclei were stained with 4', 6-Diamidino-2-phenylindole (DAPI) purchased from Sigma (St Louis, MO). Cells were then examined with an Olympus X71 fluorescence microscope.
-
The BFVL cells were plated in 12-well plates (105/well) and cultured at 37 ℃ for 12 h. Then the cells were incubated with BFV infected cells. Twenty-four hours after co-culture, the luciferase activity was measured by using Luciferase Assay System Freezer Pack (Promega, Madison, WI, USA).
Plasmids construction
Expression and purification of BFV Gag protein from E.coli
Cells and virus
Western blot (WB) assay
Immunofluorescence microscopy assay (IFA)
Mensuration of viral production
-
Gag expression plasmid pET 28a-Gag was trans-formed into competent E. coli BL21 (DE3). The expression of recombinant Gag protein was induced at 37 ℃ overnight by adding IPTG to a final concen-tration of 0.5 mmol/L to the E. coli culture. The un-induced and induced E. coli extracts were separated by SDS-PAGE, as shown in Fig. 1, a new band with molecular weight of appropriate 60 kDa was detected in the bacteria extract after IPTG induction (lane 4), but not in the un-induced bacteria extract (lane 3). The IPTG-induced cells were harvested, sonicated, and centrifuged and then the supernatant and dissolved deposit was subjected to SDS-PAGE respectively. The Gag protein band mainly appears in the dissolved deposit lane (lane 6) compared to the supernatant lane (lane 5). Thus, the prokaryotic expression Gag protein was purified by us through denaturalization.
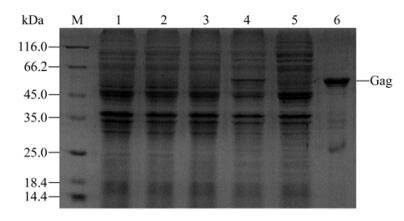
Figure 1. SDS-PAGE analysis of expression of Gag proteins in E.coli. M, molecular weight protein marker; 1, E. coli BL21 (DE3) without IPTG induction; 2, E.coli BL21 (DE3) with IPTG induction; 3, E. coli BL21 (DE3)/pET28a-Gag without IPTG induction; 4, E. coli BL21 (DE3)/pET28a-Gag with IPTG induction; 5, Supernatant of E. coli BL21 (DE3)/pET28a-Gag with IPTG induction after sonication and centrifugation; 6, Denaturalized deposit of E.coli BL21 (DE3)/pET28a-Gag with IPTG induction after sonication and centrifugation.
-
Although prokaryotic Gag protein was dissolved in denaturalization buffer, it also can be used to immunize mouse for antiserum production. Four week old BALB/c mice were immunized with denaturalized BFV Gag protein (50 μg). After five weeks (one immunization per week), the titer of antiserum was tested by ELISA. These ELISA data showed that two polyclonal anti-Gag antisera were in excess of 107. Then, the two antisera were harvested, stored in-70 ℃ and used for subsequent experiments.
-
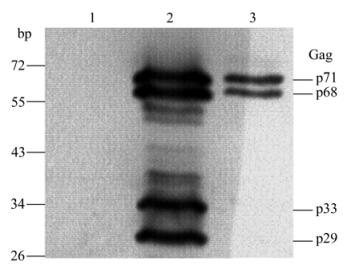
Next, the specificity of the α-Gag serum was examined by WB assay. Different numbers of BFV-infected FBL cells (104 and 105) and uninfected cells were lysed and then subjected to WB assay. As shown in Fig. 2, the α-Gag serum recognizes specifically the Gag protein in a dose dependent manner. Furthermore, the full-length Gag (p71) and its cleavages bands (p68, p33 and p29) were observed in the 105 infected cells lane (lane 2), whereas p33 and p29 cleavages bands disappeared in the 104 infected cells lane. Taken together, these data indicate that both the optimal and suboptimal cleavages occur during BFV replication and the efficiency of suboptimal cleavages is lower than optimal cleavage. Thus, these results corroborate that Gag protein of FVs needs proceeding for its eventual maturation.
-
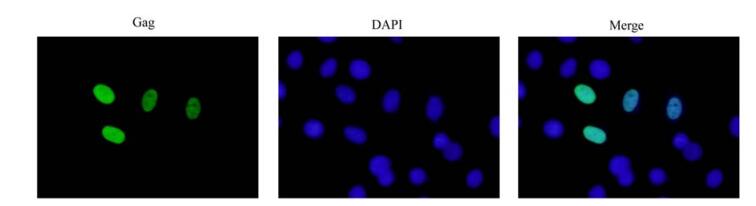
Gag protein (e.g. PFV Gag), as the late product of FVs, strictly localizes in host cellular nucleus [15]. To examine localization of BFV Gag, Gag protein was over-expressed in FBL cells through transfection with pcDNA-Gag. As shown in Fig. 3, the IFA data indicated Gag protein strictly localized in nucleus. These results imply that like HFV Gag, BFV Gag also has nuclear location signal (NLS), which causes nuclear accumulation of overproduced Gag protein.
-
In the late stage of retroviruses life cycle, the nuclear Gag protein binds the newly transcribed viral RNA genome and initialize the viral assembly. Forming the complex is essential for the viral genome and Gag protein to transport into the plasma or intracellular membrane, where they are enveloped by the Env protein and ultimately egress from the host cell [8]. To investigate Gag cellular localization and transport of the assembling viral particles into cellular membrane in the late stage of BFV life cycle, the α-Gag serum was used to perform the IFA. In our experiments, BFV-infected FBL cells were grown on a coverslip for 12 h and were co-immunostained with antibodies against tubulin (green) and Gag (Red), and the images were captured through a 100× oil immersion lens of Olympus X71 fluorescence microscope. As shown in Fig. 4, the viral late protein Gag accumulated in the nucleus of BFV infected cells, whereas the assembled viral particles distributed along MTs in cytoplasm (magnified image, right panel). These results imply that BFV may transport along cellular MTs to cellular membrane to ultimately egress from host cell. Thus, we speculate that MTs play a pivotal role in BFV replication.
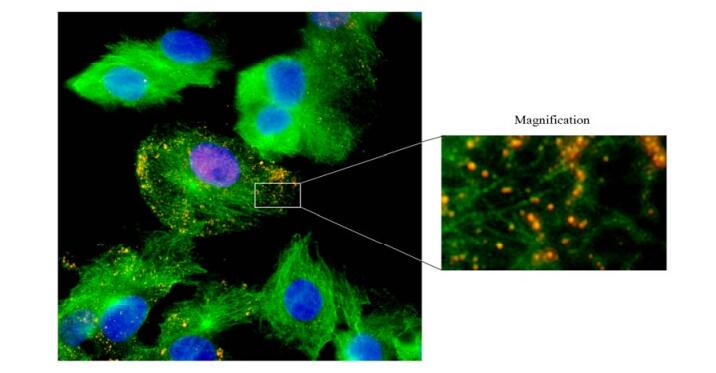
Figure 4. Subcellular distribution of BFV particles. FBL cells were infected by BFV for 12 h, and subjected to IFA using α-tubulin antibody, α-Gag serum and DAPI. The merger image (left) of MTs (green), Gag (red) and nuclei (blue) was captured through 100× oil immersion lens of Olympus X71 fluorescence microscope. Right panel: magnified part of merger image.
-
To investigate the potential involvement of MTs in BFV replication, we treated FBL cells with 1 mmol/L nocodazole, a microtubule-depolymerizing reagent, and incubated the cells with 104 BFV-infected FBL cells. Viral production was then monitored with BFVL and represented by the relative luciferase activity. We found that treatment of cells with nocodazole at 1mmol/L concentration significantly reduced about 80% of viral infectivity, as correlated with the decrease in the relative luciferase activity (Fig. 5A). Meanwhile no obvious toxicity was detected by measuring cell viability upon nocodazole treatment (Fig. 5B). These results indicate that MTs are possibly required for efficient replication of BFV.
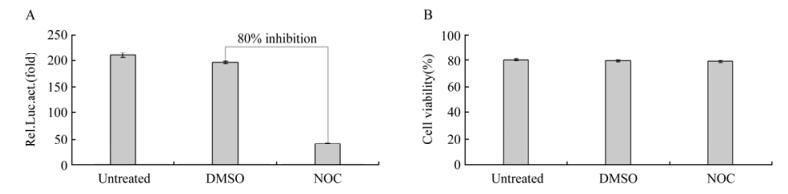
Figure 5. BFV replicaton was inhibited by nocodazole. A. FBL cells were treated with 1 µM nocodazole or equivalent volume of DMSO for 2 h and co-incubated with 104 BFV-infected FBL cells for 24 h. Then viral production was monitored with BFVL by the relative luciferase activity. B. FBL cells were treated with DMSO or nocodazole for 2 h, and cell viability was analyzed by trypan blue exclusion assay.
Expression and purification of BFV Gag protein
Preparation of α-Gag serum
Specificity analysis of α-Gag serum by WB assay
Detection of Gag protein by IFA
BFV particles subcellular distribution
MTs are required for BFV replication
-
Foamy viruses (FVs), the oldest known genus of Retroviridae, are unique among the retroviruses, which are only genus of the Spumaretrovirinae subfamily. Heretofore, it was considered that FVs are nonpathogenic and do not cause disease in naturally or experimentally infected animals [10]. Since they have no disease association, FVs are being exploited as vectors for gene deliver applications. On the other hand, despite having no disease association, it remains unknown whether FVs induce any pathology in immunosuppressed hosts. Therefore, integrated evaluation of risk of FVs-based gene deliver is necessary before its applications. Taken together, despite advances in understanding the molecular biology of FVs replication, it will require an understanding of all aspects of FVs biology such as virus-host dynamics.
Investigation into cellular trafficking of FVs provides an attractive view of virus-host dynamics. Despite a growing body of knowledge accumulating in this field, how FVs trafficking in host cells is still poorly understood. Many viruses require MTs during cell entry for efficient nuclear targeting, either for cytosolic transport of naked viral particles [6, 16, 20]. In addition, there is an growing list of reports showing that MT motors also catalyze the intracellular transport of many viral structures through direct interaction of viral components with MT motors [17]. Here, our results indicate BFV transports along MTs that are required for efficient viral replication. It not only confirms the discovery on HFV cellular trafficking, but also sheds a new light on BFV virus-host dynamics. Viruses must engage the bidirectional cellular transport mechanism for completing its whole life cycle, and a particular motor moves only in one direction, the cell-specific organization of the MT determines the destination a specific motor can reach. Heretofore, it is not clear that which components of MTs involve in BFV bidirectional cellular transport. Thus it really needs to investigate the direct interact-tion between Gag and MT components in our subsequent research.
As far as we know, when comparing individual PFV cleavage efficiency sites with each other, the broad range of cleavage efficiency of an individual site is remarkable. The HFV Gag p68/p3 is efficiently cleaved, most other sites are cut at an immediate or even lower rate [13]. The efficiency of optimal cleavage ranges from 50% to nearly 100%, in contrast, the suboptimal cleavages are only observed when sensitive methods of detection are applied and not found in every cell type analyzed. In the present study, four cleavage bands, p71, p68, p33 and p29, were observed in lane 2, but the p33 and p29 cleavage bands disappeared in lane 3 (Fig. 2). These results indicate that both the optimal and suboptimal cleavages occur during BFV infection of FBL cells, and the Gag p68/p3 cleavage is most efficient among all cleavages.













 DownLoad:
DownLoad: