-
Human immunodeficiency virus type-1 (HIV-1) infection leads to a defined pattern of disease progression and causes clinical immunodeficiency syndrome in an average time of 9 to 10 years [1]. However, some individuals, who are called long-term non-progressors (LTNP), can remain asymptomatic and maintain stable CD4+ lymphocyte numbers > 500/mm3 for more than 10 years [2, 4]. Investigation of the factors contributing to the delay of disease progression in such individuals holds promise for intervention and prevention of disease.
Many studies have been completed in the past few years to explore various mechanisms for the control of disease progression in HIV-1 infected people, including neutralizing antibodies. However, a lot of the data is controversial, especially that from studies on neutralizing antibodies. Some researchers believed that the neutralizing antibody plays an important role in controlling disease progression, because they found that the plasma/sera from HIV-1 infected LTNPs displayed a broader neutralization spectrum against different subtypes of HIV-1 than those from the infected individuals in the other stages [3, 4, 6]. However, contrary to those data, others studies demonstrated that even cross-reactive neutralizing humoral immunity couldn't protect an infected individual from HIV-1 disease progression [5]. In this paper, we examined the correlation between broad neutralizing antibodies and disease progression in HIV-1 infected Chinese. Neutralizing antibodies were evaluated by using several pseudoviruses expressing Env proteins of HIV variants prevalent both in China and abroad separately. 11 long-term non-progressors (LTNP) and 9 typical progressors (TP) were screened and enrolled into the study with informed consent using the following criteria: (1) LTNPs had been infected with HIV for more than 10 years with CD4 cell numbers always more than 500cells/μL although all of them were treatment-naïve; (2) TPs were individuals infected by HIV-1 at the same time as LTNPs and were also treatment-naive but with CD4 cell numbers less than 200 and had not taken any immuno modulatory agents in the last one year before being enrolled into the study. The clinical data is listed in Table 1. All of the subjects were infected by the practice of unsafe commercial blood donation in the late 1980's.

Table 1. Clincal information of enrolled participants
HTML
-
Plasma was collected from LTNPs and TPs. Subtypes of variants were determined by phylogenetic analysis based on sequences of full-length gag, pol and partial env genes acquired by RT-PCR. Clade Thai B was revealed as the only subtype in both populations as reported previously (data is not shown).
-
Neutralizing antibody against HIV-1 pseudoviruses was tested using the standard luciferase reporter gene assay in TZM-bl cells. In brief, plasma was heat inactivated at 56℃ for half an hour and serially diluted with DMEM medium containing 10% FBS into 96-well plates. 200×50% tissue culture infectious doses (TCID50) pseudoviruses were then added to each well and co-cultured with serially diluted plasma for 1 h at 37℃ before 1×104 TZM-bl cells were added. The cells were cultured for 48 h before being lysed. Promega luciferase assay substrate was then used to analyze luciferase activity on a Luciferase reader (Perkin Elmer, USA). All samples were tested in triplicate, ID50 values were calculated with respect to the formula: (detection serum–negative control serum) %/(negative control serum–cell only). Plasma from healthy individuals was used as the negative control.
-
To check the correlations between neutralizing antibody and binding antibody, the amount of binding antibody targeting HIV-1 gp120 protein was measured. To do this, gp120 antigens of Thai-B CNHN24 were expressed from transiently transfected 293T cell supernatants and coated 96 well microtiter plates (Corning, USA) as described before [8]. After wells were blocked with 200 µL/well of blocking buffer (4% milk-whey in PBS) overnight, 100 µL of serially diluted plasma were added to duplicate wells and incubated for 1 h. Following the antibody incubation, 100 µL of biotinylated anti-human IgG at 1:1000 dilution was added to each well and incubated for 1 h, horseradish-peroxiadase (HRP)-conjugated streptavidin at 1:2000 dilution was then added (100 µL/well) and incubated for 1 h. Between each step, the plates were washed 5 times with washing buffer (PBS at pH 7.2 with 0.1% triton X-100). Finally, 100 µL/well of fresh TMB substrate (Sigma, USA) was added and incubated for 3.5 minutes. The reaction was stopped by adding 50 µL of 2 mol/L H2SO4. The absorption/ optical density (OD) was measured at a wavelength of 450 nm. To check the possible difference of binding antibody in LTNP and TP, antibody titers were compared.
-
Statistical analyses were performed using SPSS V.10.0 software. T-test, Pearson χ2 test and Fisher's exact test were used to analyse the different issues between LTNPs and TPs, and the correlations were also assessed. All statistical analyses were two sided, and p < 0.05 was considered statistically significant.
Plasma samples
Test of neutralizing antibodies
Test of binding antibody
Statistical analysis
-
4LTNPs who showed Viral Load (VL) < LDL had higher CD4+ counts with an average value equal to 649.5. This value was less than the average value of 730 for all the LTNPs. 6 clade B and 3 clade C pseudoviruses were used to evaluate the neutralization profile of the samples from HIV-1 infected drug-naïve individuals (Table 2). It seemed that the plasmas from the Thai-B infected individuals were better at neutralizing clade B pseudoviruses compared with the clade C pseudoviruses. 5 out of 20 plasma could neutralize only 1 out of 3 clade C pseudoviruses, 1 plasma could neutralize 2 out of 3 clade C pseudoviruses, and only 1 plasma could neutralize all of 3 clade C pseudoviruses. The broad-spectrum neutralization activity of plasma from LTNP and TP was compared by using correlation (Pearson Method) analysis. The result (r=0.17, t=0.073, p > 0.05) indicated there was no significant difference has been observed between the two groups (Fig. 1A). The cross-reactive neutralizing activity of neutralizing antibodies in LTNP was found in some previous studies, so we checked the ability of our plasma to neutralize at least 1 kind of clade C pseudovirus. No significant difference was found in the CD4+ T cell count and viral load between the cross-reactive neutralizing activity group and the no cross-reactive neutralizing activity group (Fig. 2A and B).

Table 2. Neutralization activities (ID50) of plasma samples against different pseudotyped viruses
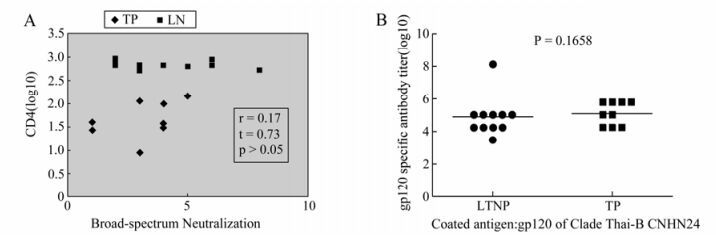
Figure 1. Investigation of neutralizing activity and gp120 specific binding antibody titer between the LTNPs and TPs. No difference on neutralizing activity (A) and gp120 specific binding antibody titer (B) was found between the LTNP and TP. A: Neutralization profile of the samples from LTNP and TP were tested with 6 clade B and 3 clade C pseudoviruses. The broad-spectrum neutralization activity was compared by using Correlation Analysis (Pearson Method). B: Level of binding antibody to HIV-1 gp120 protein was measured with expressed gp120 protein in the lab. T-test was used to compare the binding antibody titer.
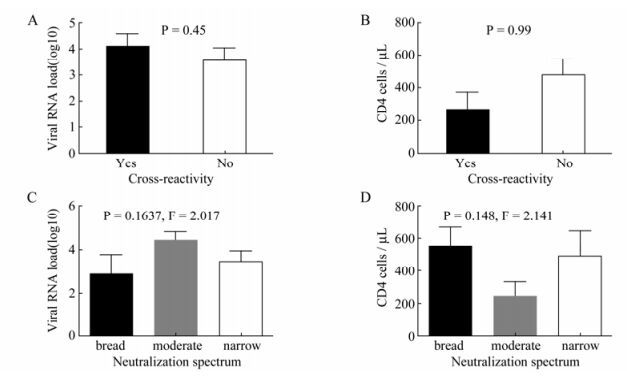
Figure 2. Neutralization activity has no protective effect on viral load and CD4+ T count. Correlations of Cross-clade neutralization activity with viral load (A) and CD4+ T count (B) were analyzed. Samples with cross-reactivity were defined as plasma that could neutralize clade C pseudoviruses although they were infected with subtype B virus. Correlations of neutralization spectrum with viral load (C) and CD4+ T count (D) were listed below. Plasma that could neutralize more than 5 out of 9 pseudo-viruses was considered as a broad neutralization spectrum, while the plasma which could neutralize 3-5 pseudoviruses was considered to be a moderate spectrum and the plasma which could neutralize less than 3 pseudoviruses was considered a narrow neutralization spectrum.
Breadth of neutralizing antibody is another characteristic which may affect disease progression of HIV-1 infected people. We assigned the plasma which could neutralize more than 5 out of 9 pseudo-viruses as the broad neutralization spectrum, the plasma which could neutralize 3-5 pseudoviruses as the moderate spectrum and the plasma which could neutralize less than 3 pseudoviruses as the narrow neutralization spectrum. No statistically significant differences in the CD4+ T cell count or viral load of these 3 groups were observed (Fig. 2C and D).
The end titer was 2-fold higher than the OD450 value of the negative control wells with the normal human plasma. The gp120 specific antibody titers were compared in LTNP and TP, and no statistical significance was found (Fig. 1B), suggesting the two groups possess a similar Env specific humoral response.
Correlations between binding antibody and neutralizing antibody were further compared. Pseudovirus SF162 was very sensitive to the plasma of HIV-1 infected individuals, especially to the V3 antibodies [7], which was also demonstrated in our study. So neutralizing antibody targeting SF162 was used as representative of total neutralizing antibody. No statistical correlation was observed also (Fig. 3). The result suggests that the gp120-specific antibodies titer is not correlated to the anti-SF162 neutralizing antibody titer.
-
In China, Thai-B was the dominant subtype in former plasma donors in China. Studying the neutralizing ability of plasma from different progressors infected by clade Thai-B can provide unique insight into differentiating the neutralizing activity of antibodies elicited by Env glycoproteins, and understanding the mechanisms of the Env specific immune response will aid the rational design of Env based vaccines. Antibodies against Env protein can be detected a few weeks after HIV-1 infection. With time, a broader humoral reactivity can develop in some individuals [1, 9]. However, most of the antibodies developed in HIV-1 infected subjects demonstrate no protective activity. The data in this report addresses an important question in the protective function of plasma from LTNP and TP. In our research, the plasma of LTNP do not display stronger neutralization activity than those of TP, and a lack of association between NAb and either serum viral load or CD4 +T cell count is also observed. These results are consistent with the latest reports.
Taken together, albeit the sample size involved in this research is relatively small, our results provide some insight into the relationship between the neutralizing antibody and disease progression. Nevertheless, the experiments described above further prove that NAbs may not play a role in delaying disease progression. A larger sample size would help to confirm the conclusion with stronger evidence.














 DownLoad:
DownLoad: