-
Enterovirus 71 (EV71) was first isolated and identified in 1969 in California USA. It is the most frequently occurring pathogen in hand-foot-and-mouth disease (HFMD) and symptoms include neurological dysfunction, such as aseptic meningitis and poliomyelitis-like paralysis [10]. It was reported that EV71 was responsible for the HFMD outbreaks in Taiwan, Japan and Singapore. Moreover, large outbreaks have occurred several times in mainland China since 2008. There have been more than 1.5 million HFMD cases, and 57.5% of the deaths were caused by EV 71. On average, EV71 has been the cause of about 1000 deaths every year. Children younger than 5 years old have been found to be particularly susceptible to EV71 and the associated neurological disease and fatalities [15]. In conclusion, EV71 infection has been regarded as an important public health problem which has the potential to cause serious clinical illness and death in young children [6, 19].
Enterovirus 71, classified as a species of genus Enterovirus in the Picornaviridae family, is a small, nonenveloped, icosahedral RNA virus. It has a single-stranded RNA genome of approximately 7500 nucleotides [16] which is a single open reading frame (ORF) flanked by a 5'-untranslated region (5'UTR) and a 3'-untrans-lated region (3' UTR). The ORF is translated into a large polyprotein and it can be cleaved into P1, P2, and P3 regions. The P1 region encodes four structural proteins VP1, VP2, VP3 and VP4. The P2 and P3 regions encode nonstructural proteins, such as proteases 2A, 2B, and 3CD, which are responsible for virus replication and virulence. These proteins spontaneously assemble and form the crystalline virus-like particles [8].
The development of an EV71 vaccine is urgent and indispensable for the prevention and control of HFMD. Previous research has reported various types of EV71 vaccines, including a VP1 peptide vaccine, a DNA vaccine, and attenuated and inactivated vaccines [13, 17, 20]. Based on the enterovirus vaccine development, an attenuated and inactivated vaccine appears more feasible. However, a protein engineering approach may be more applicable. In this study, the EV71-P1 gene was processed and cloned into the eukaryotic expression vector pPIC9k and the resulting vector could manufacture and secret EV71-P1 protein. The soluble EV71 P1 protein was purified by column chromatography and was then identified by Western blot analysis. The results of immunogenicity analysis showed that the EV71 P1 protein had excellent immunogenicity and could stimulate the production of EV71-VP1 IgG antibody in injected rabbits.
HTML
-
The P. pastoris strain was histidine requiring auxotroph GS115 (his4) and was purchased from Invitrogen. The SYBR Green Real-time Master Mix Taq Polymerase was made by TOYOBO and the chromatography column was purchased from Pharmacia. The Rabbit EV71-vp1 monoclonal antibody was made by Shanghai Jiya Biology Company, China. The HRP-conjugated goat anti-rabbit IgG secondary antibody was made by Beijing Zhongshan Biology Company, China.
-
The EV71 P1 gene was derived from EV71 C4-subtype strain. After codon optimization, the EV71-P1 gene was synthesized and then cloned into pPIC9k expression vector by Shanghai Genery Biotechnology Company.
-
The pPIC9k-EV71 P1 was transformed into the P. pastoris GS115 by electroporation and the transformants were initially screened for their viability in the medium without histidine. The positive recombinants were transferred in a Yeast extract Peptone dextrose medium (YPD) plant with a G418 (Neomycin) gradient concentration from 0.5 to 5.0 mg/mL respectively and incubated in 28℃ for 7 d. The genomic DNA of recombinant with high G-418 resistance was extracted by a yeast genomic DNA purification kit (Biotaq) and then was quantified by OD260nm. The yeast genomic DNA temple for each PCR sample was 1 ng. Amplification was performed by the primer P1S: 5'-ccgaattcatg ggttctcaggtt-'3; P1A 5'-aagacaaagcggccgccaatgt agtgatagcggttc-'3, (General biotechnology company) as follows: 10 min at 94℃ and 25 cycles consisting of 30 s at 94℃, 30 s at 55℃, 90 s at 72℃, 20 min at 72℃.
-
Real-time quantitative PCR was used to quantify the EV71-P1 gene copy level in high G418-resistant recombinants. The PCR reaction was performed by the primers: PS: 5'-ccagccaaaactatttg-3', PA5'-aatgtagtgatagcggttcgac-3', (Generay biotechnology company) on an Eppendorf Realplex2 Mastercycler (Eppendorf) according to the manufacturer's instructions. Each PCR reaction was performed on 1 pg of yeast genomic DNA mixed with 2.5 µL of Master Mix Taq Polymerase (Tiangen), 2.5 µL of SYBR Green I, 100 pg of each primer for a final volume of 25 µL. Amplifications were performed as follows: 10 min at 94℃ and 35 cycles consisting of 10 s at 94℃, 30 s at 50℃, 30 s at 72℃.
-
The recombinants were cultured in Buffered Glycerol-complex Medium (BMGY) at 30℃ and then resuspended in Buffered Methanol-complex Medium (BMMY). The cultures were shaken at 250 r/min for 108 h at 29℃, and the methanol concentration retained 0.5% (w/v).
-
The supernatant was harvested by 3600 rpm centrifugation for 5 min and concentrated 10-fold by PEG6000, dialyzed with buffer Ⅰ (20 mmol/L tris, 50 mmol/L NaCl, pH 7.8) overnight and then applied to the DEAE-Sepharose Fast Flow (DEAE-SFF) column which was pre-equilibrated with buffer Ⅰ. The adsorbed protein was eluted by gradient NaCl (0.1–1 mol/L) in 20 mmol/L Tris-buffer (pH 7.8). The components were collected and dialyzed in buffer Ⅱ (20 mmol/L Tris-Cl buffer, pH 7.8) at 4℃ overnight.
-
10% SDS-PAGE was used for electrophoresis. For Western blotting, the protein was then transferred to nitrocellulose membranes. Rabbit EV71-vp1 monoclonal antibody and HRP-conjugated goat anti-rabbit IgG secondary antibody were used to detect the EV71-P1 protein.
-
The antigenicity of EV71-P1 was determined by ELISA. 100 ng purified P1 protein was used as the coating antigens for each well. After blocking and washing, 100 µL of the negative or positive EV71 antibody sample (EV71VP1 monoclonal antibody or EV71 antibody positive serum) were added in duplicate into individual wells and incubated for 1 h at room temperature. Reactions were detected using HRP-conjugated second antibody and then were measured by an ELISA microplate reader at OD490nm, the test was repeated in parallel three times.
-
All animal experiments in this study were approved by the Animal Care and Use Committee, Jianghan University (Wuhan, Hubei). Rabbits were subcutaneously injected with 20 µg purified EV71-P1 protein. The protein was emulsified with Freund's complete adjuvant (Sigma) for primary injection and Freund's incomplete adjuvant (Sigma) for booster injections. The booster injections were given after 8 days of primary immunization. Rabbit's serum before the primary injection was used as the negative control. The presence of VP1 IgG antibodies in serum was determined by ELISA which used the purified VP1 protein (produced by E.coli) as the coating antigen. Following blocking and washing, 100 µL of the collected and negative control serum (1:100 dilution) were added for primary antibodies.
Strain and reagent
Gene processing and Construction of Expression Vector
Selection of Positive Recombinants
Real-Time PCR
Expression of EV71-P1 protein
Column chromatography
SDS-PAGE and Western blot
ELISA analysis
Immunization of rabbits
-
The EV71 P1 gene with codon optimization was synthesized by the Shanghai Generay Biotechnology Company was about 2.0 kb and further confirmed by sequencing (data not shown).
-
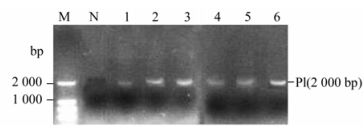
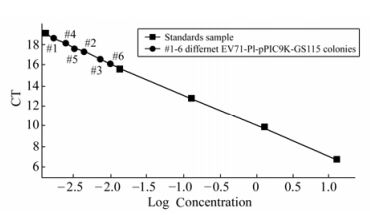
The recombinant P. pastoris GS115 strains were selected according to their G418 resistance. The result showing the highest resistance to G418 was 4.0 mg/mL. The genomic DNA of the each G418 resistance strains were respectively selected for the template by colony PCR and real time PCR. Results from the colony PCR (Fig. 1) showed the EV71-P1 gene with 2000 bp could be detected in each high G418 resistance recombinant. The real time PCR showed that isolate #6 with 4.0 mg/mL G418 resistance had the highest copy of the gene of EV71-P1 (Fig. 2) and was selected for further study.
-
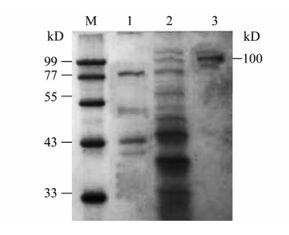
The expression and purification of EV71-P1 protein was carried out as described in the methods. The protein was identified to be about 100 kD by SDS-PAGE (Fig. 3) and it was further confirmed by Western blot analysis with the EV71 antibody (Fig. 4).The SDS-PAGE result showed the EV71-P1 protein was eluted with 350 mmol/L NaCl buffer (data not shown).
-
ELISA was used to detect the antigenicity of the EV71-P1 protein. The result showed that the reaction between the EV71-P1 protein and the EV71-VP1 monoclonal antibody was stronger than the one between the EV71-P1 protein and the EV71 antibody positive serum (Fig. 5).
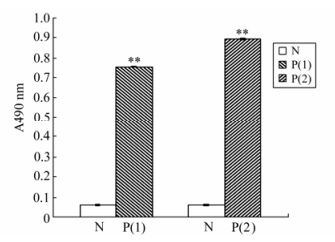
Figure 5. ELISA analysis of EV71-P1 with different primary antibody. The coating antigen was 100 ng purified EV71-P1 protein and the result was observed in the OD490nm with OPD. N group: the primary antibody was humanV71 antibody negative serum; P(1) group: the primary antibody was EV71vp1 monoclonal antibody; P(2) group: the primary antibody was human EV71 antibody positive serum. **p < 0.01
-
To determine the immunogenicity of the EV71-P1 protein, immunization of rabbits was performed. Results of an indirect ELISA on the collected sera using purified VP1 (produced by E.coli) as a coating antigen. The result showed the VP1 IgG antibody could be detected in rabbits' serum on day 20 after primary immunization with a dilution of 1:100. As expected, pre-immune serum showed no reactivity with the VP1 protein (Fig. 6).
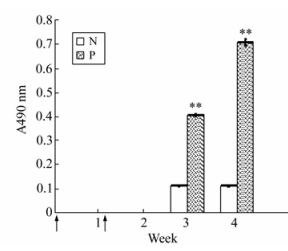
Figure 6. The mean of anti-EV71 IgG antibody titers in the rabbit injected EV71-P1 protein.The coating antigen was EV71-VP1 protein and the result was observed in the OD490nm with OPD. Arrows indicate immunization time points. N group: the primary antibody was rabbit serum in preimmune; P group: the primary antibody was rabbit serum in postimmune 20ug P1 protein. **p < 0.01
Gene processing and Construction of Expression Vector
The Selection of a high producing recombinant P. pastoris strain
Production and purification of EV71-P1 protein
Analysis of the immunogenicity of EV71-P1 protein
Induction of specific antibodies in rabbit
-
Several waves of global outbreaks of EV71 infection have been recorded over the last three decades [18]and recent active circulation of EV71 is evident in China. However, there is no specific treatment available for EV71 infection [11]and a safe and effective vaccine is urgently needed. There have been some studies focusing on the EV71 nucleocapsid protein which have identified some epitopes and neutralizing sites in the VP1 and VP2 proteins [12]. Immunization of mice treated with the VP1 recombinant protein resulted in a mixed T1 and T2 response [21]. EV71 VP1-containing milk could stimulate the antibody neutralization in mice [2]. Recently, the poly nucleocapsid protein has become the focus of vaccine research, and the combined peptides in the VP2, VP3 and VP1 domain have been shown to provide effective protection against EV71 [14]. The induction of Th1 and Th2 immune responses was observed in mouse by vaccination with the P1 protein virus-like particles [5]. The above research showed that the P1 protein including VP1, VP2, VP3, VP4 protein could be an ideal candidate for the EV71 vaccine.
There are several new EV71 candidate vaccines that have been developed and evaluated recently. These candidates include an avirulent EV71 vaccine, an EV71-like particle vaccine, a DNA vaccine and a recombinant VP1 protein/milk vaccine [3, 4, 7, 21]. All of these vaccines showed good immune responses in vaccinated mice and the maternal antibody induced by the EV71-like particle vaccine could protect neonatal mice from EV71 challenge [5, 22]. However, the cost of these reported vaccines is too high to be used in the vast rural areas in China which represents the high risk regions of EV71 infection. Therefore, further research is needed to develop an efficient and economical vaccine. Pichia pastoris is a powerful and economical expression system for the production of functional recombinant proteins. This system combines the advantages of high expression levels, low cost, capacity of the post-translational modifications characteristic of higher eukaryotes [1, 9] and the recombinant glycosylated protein produced from P. pastoris is an ideal medicinal candidate [9].
In this report, we cloned, expressed and purified EV71-P1 protein in P.pastoris and the production of EV71-P1 protein was effective and efficiently secreted. Our production system had the following characteristics. (1) We designed and processed the EV71-P1 gene for effective secretary expression of EV71-P1 protein in P. pastoris with its original antigenicity; (2) the concentration control of methanol was very important, 0.5% (W/V) methanol concentration was suitable for the EV71-P1 protein expression in our study; (3) the EV71-P1 protein could be detected after 48 h induction and the production increased continuously until 108 h (data not shown); (4) The yield of EV71-P1 protein was about 8 mg/L in the shaker flask with a yeast concentration of 120 g/L, we will optimize the fermentation conditions for a fermentation tank in future research; (5) The purification step was effective and simple and the recovery efficiency was about 70%.
The EV71-P1 protein molecular weight was 75 kD, however the SDS-PAGE and Western blot results showed the target protein was about 100kD. There are more than 10 glycosylation sites in the EV71-P1 protein and some glycosylation sites are nearby the highly antigenic region. We speculate that some glycosylation sites made the EV71-P1 protein weight increase. The ELISA result showed that the EV71-P1 glycoprotein reacted with the human EV71 antibody positive serum stronger than the EV71 monoclonal antibody. These data suggest that there are some important antigenic domains in the P1 protein beside the VP1 domain, and the VP2, VP3, VP4 protein should be included in the vaccine design. The strong antibody responses induced by immunization indicated good immunogenicity of the protein and additional studies such as neutralization and viral challenge should be performed in further research to evaluate the protective efficacy of EV71-P1 protein against EV71 infection.
This is the first report about the expression and purification of EV71-P1 protein in P. pastoris and the simple and economic way in which we produced and purified the protein is appropriate to large-scale production. The EV71-P1 protein showed strong immunogenicity and stimulated the VP1 special IgG antibody in the injected rabbit. Therefore, we suggested that EV71-P1 protein is an ideal candidate for EV71 vaccine.














 DownLoad:
DownLoad: