HTML
-
Porcine circovirus 2 (PCV2) is the causative agent of postweaning multisystemic wasting syndrome (PMWS), a multifactorial disease that causes severe growth retardation, wasting and increased mortality in weaned pigs (Segalés J, 2012). PCV2 has additionally been isolated from aborted, mummified foetuses and nonviable neonatal piglets (Meehan B M, 2001; Brunborg I M, 2007). PCV2 strains are divided into two major genotypes, PCV2a and PCV2b (Segalés J, 2012), with significant antigenic homology between different strains (McNeilly F, 2001).
Several studies have attempted to experimentally reproduce PMWS by inoculating pigs with PCV2, which mainly led to subclinical infections (reviewed by Segalés J, 2012). At present, scientists working in basic and applied research fields, as well as vaccine development, have problems in studying the pathogenesis of PMWS and designing appropriate vaccine strategies due to the absence of a highly reproducible PMWS model.
Recent studies demonstrated that in utero PCV2 infection during insemination (SarliG, 2012) or late gestation (Ha Y, 2008) results in live-born infected piglets highly susceptible to disease upon co-infection with other pathogens or immunostimulation (Ha Y, 2008). Two different PCV2 sequences had been identified in one pig (Lefebvre D J, 2008; Gerber P F, 2013). The PCV2 strain, VC2002, was originally isolated from an inguinal lymph node of a Belgian PMWS-affected pig (Meerts P, 2004). Our group subsequently discovered that VC2002 was a mixture of two PCV2b viruses, K2 and K39, displaying 94% identity at the nucleotide and amino acid levels (Lefebvre D J, 2008). Preliminary analysis of K2 and K39 in porcine foetuses inoculated at 55 days of gestation revealed different biological characteristics (Saha D and Nauwynck HJ, unpublished results). Notably, K39 induced pathology in foetuses collected at 21 days post-inoculation(76 days of gestation), whereas no pathology was evident in K2-infected foetuses. This finding prompted the hypothesis that in vivo infection of embryos/foetuses with the comparatively less foetopathogenic PCV2, K2, at early stages of gestation (before the development of immunocompetence) confers a status of immunotolerance at birth. Postnatal super-infection of such immunotolerant animals with the highly foetopathogenic PCV2, K39, should therefore cause disease and no recognition by the immune system.
The aim of the present study was to examine the above theory by generating PCV2-immunotolerant pigs via intra-uterine inoculation of K2 into immuno-incompetent porcine foetuses and analysing the outcome of postnatal super-infection with K39 after birth.
-
Two different PK-15-adapted PCV2b strains, K2 (EF990645) and K39 (EF990646) (Lefebvre D J, 2008), were used. PCV-negative PK-15 cells were grown in minimal essential medium(MEM)containing Earle's salts and GlutaMAXTM -Ⅰ (MEM+GlutaMAXTM -Ⅰ, Gibco, Grand Island, USA) and supplemented with 5% foetal calf serum(FCS), 100 U/mL penicillin, 0.1 mg/mL streptomycin and 0.1 mg/mL kanamycin. Cell cultures were maintained at 37℃ in the presence of 5% CO2 (Saha D, 2010).
-
At 55 days of gestation, three conventional PCV2-seropositive Landrace sows (S1, S2 and S3) were subjected to laparotomy as described earlier by Saha D (2010) (Table 1). Three foetuses of each sow were inoculated: one with K2-104.3 TCID50, one with K39-104.3 TCID50 and one with PK-15 cell culture medium. Inoculation and operative procedures were performed according to the above report (Saha D, 2010). Sows were housed separately, and clinical signs monitored as exactly as described inSaha D (2010). At 21 days post inoculation (dpi), sows were humanely euthanized. All foetuses were examined for gross lesions. Foetal tissue samples were obtained for histopathological examination, virus titration and indirect immunofl uorescence analyses (Saha D, 2010). Serum and abdominal fluid from all foetuses and pre-serum (before laparotomy) and post-serum (at the time of euthanasia) of sows were collected.
Sow no. Foetus no.* PCV2 strain PCV2 replication Heart Lungs Spleen Liver Kidneys Thymus Tonsils Ileum Cerebrum VT IIF VT IIF VT IIF VT IIF VT IIF VT IIF VT IIF VT IIF VT IIF S1 L1 K2 6.3 900 3.0 253 5.7 NA 5.2 10353 2.7 587 5.6 NA 3.7 NA 4.1 NA 2.0 10 S2 R3 K2 5.5 976 4.7 2363 6.3 23803 5.3 12110 5.0 2890 5.8 7073 4.8 3416 4.3 3850 2.5 13 S3 L2 K2 4.0 580 3.5 1260 5.6 8320 5.0 2337 2.5 880 4.8 10383 4.1 1297 4.8 670 2.5 17 S1 L2 K39 6.0 3550 2.5 223 4.5 1160 2.8 1337 < 1.7 10 3.0 11883 3.8 13 1.8 63 < 1.7 20 S2 L2 K39 6.0 4027 2.2 3 3.5 80 3.9 1297 2.1 27 3.0 30 2.8 37 3.0 10 < 1.7 0 S3 L3 K39 5.8 4350 3.0 20 3.8 163 4.3 1137 2.7 3 4.3 NA 2.8 NA 1.8 NA 2.5 0 * L = left horn; R = right horn. Numbering of foetuses is in sequence from ovary to cervix.
VT = Virus titres (log10 TCID50/g); IIF = Indirect immunofluorescence staining (number of PCV2-positive cells /10 mm2 ); NA = not availableTable 1. Virus replication in different foetal organs after intra-foetal inoculation with K2 or K39 at 55 days of gestation, collected at 21 days post-inoculation
-
Four conventional PCV2-seropositive Landrace sows were subjected to laparotomy on the left side of the abdomen at 45 days of gestation. Six foetuses of each sow were inoculated (three with K2 and three mockinoculated with PK-15 cell culture medium). In the first two sows (S4 and S5), six foetuses (three for each sow) were inoculated with a K2-high dose-104.3 TCID50/foetus, whereas six foetuses (three for each sow) from the other two sows (S6 and S7) were inoculated with a K2-low dose-102.3 TCID50/foetus (Table 2). The procedures of laparotomy and intra-foetal inoculation of virus or medium were identical to those described previously (Saha D, 2010). At 69 days post inoculation (114 days of gestation), a second laparotomy procedure was carried out on the right side of the abdomen, following which all foetuses were collected (Table 2). Proper care was taken during collection of foetuses to avoid any possibility of PCV2 spread from the virus-inoculated to mockinoculated foetuses. Each of the collected newborn piglets was housed individually in separate isolators.
Sow no. Numbering of pigs at birth Intra-foetal inoculation with Dose
(log10 TCID50)Clinical outcome at birth (69 dpi) S4 F1 K2 4.3 live F2 K2 4.3 mummified F3 K2 4.3 mummified F4 mock - live F5 mock - live F6 mock - live S5 F1 K2 4.3 mummified F2 K2 4.3 mummified F3 K2 4.3 mummified F4 mock - live F5 mock - live F6 mock - live S6* F1 K2 2.3 live F2 K2 2.3 mummified F3 K2 2.3 mummified F4 mock - live F5 mock - live F6 mock - live S7* F1 K2 2.3 mummified F2 K2 2.3 mummified F3 K2 2.3 mummified F4 mock - live F5 mock - live F6 mock - live * live-born pigs from sows S6 and S7 were immunostimulated. Table 2. Clinical outcome of pigs inoculated intra-foetally with PCV2-K2 or mock-inoculated at 45 days of gestation and collected at 69 days post-inoculation (at birth)
Blood was collected at birth from the umbilical cord of K2-inoculated and mock-inoculated piglets. Serum samples of the sows were obtained prior to the fi rst (preserum) and second laparotomy (post-serum) procedures.
-
Live-born K2-and mock-inoculated colostrum-deprived, caesarean-derived (CD/CD) piglets from S4 and S5 or S6 and S7 were oronasally super-inoculated with either K39-104.3 TCID50/pig or medium at days 6 and 8, respectively. Piglets from S6 and S7 were vaccinated against parvovirus (Parvoject, Merial, Belgium) on day 2. Piglets were monitored daily for PMWS-associated symptoms(Segalés J, 2012). Half of the left and right inguinal lymph nodes (ILNs) were biopsied at 14 and 21 days post super-inoculation (dpsi), respectively (Meerts P, 2005) and the remaining ILNs collected at euthanasia (28 dpsi). All pigs were bled at 0, 7, 14, 21 and 28 dpsi.
Tissue suspensions (10% or 20% wt/vol) were prepared from collected organs, and PCV2 titres determined. PCV2-positive cell numbers in different organs were estimated using indirect immunofluorescence staining(IIF) (Saha D, 2010). For IIF, anti-PCV2 monoclonal antibodies (mAbs) F190 (1:500), F217 (1:100) (McNeilly F, 2001) or biotin-conjugated anti-PCV2 polyclonal antibodies (pAbs) (1:50) in PBS and fl uorescein isothiocyanate(FITC)-labelled goat-anti-mouse pAbs (Molecular Probes, USA) or FITC-labelled streptavidin (1:200) (Molecular Probes) in PBS were used as primary and secondary antibodies, respectively. The number of PCV2-positive cells per 10 mm2 of tissue was determined (Saha D, 2010).
To differentiate between K2 and K39, double immunofluorescence staining was performed in ILNs (Saha D, 2011). The mAbs 114C8 (IgG1; reacts with K2 and K39) and 16G12 (IgG2a; reacts with K39) diluted 1:10 (Saha D, 2012a) were used as primary and 1:200 diluted mAbs FITC-labelled goat-anti-mouse IgG1 and goatanti-mouse IgG2a Alexa-Flour 594 (Molecular Probes) as secondary antibodies. The number of PCV2-positive cells was determined as above. DNA was extracted from heart and lung tissue suspensions of PCV2-inoculated and adjacent foetuses (21 dpi) as well as piglet sera (69 dpi) (Saha D, 2010). Amplification and sequencing of the PCV2 capsid gene were performed in keeping with previous reports (Saha D, 2010; Saha D, 2012b).
PCV2-specific Ab titres in serum or abdominal fl uids of foetuses were determined with the immuno-peroxidase monolayer assay (IPMA) (Saha D, 2010; Saha D, 2014). Sow antibody titres against PCV2 and PRRSV were assessed with IPMA and against porcine parvovirus (PPV) with the hemagglutination inhibition (HI) test (Saha D, 2010). PCV2-specific IPMA Abs in sera of piglets were collected at birth and 0, 7, 14, 21 and 28 dpsi. PCV2-neutralising Abs against K2 or K39 were determined using the classical or sensitive neutralisation assay (Meerts P, 2005; Saha D, 2012c).
Viruses and cells
Short-term outcome (21 days post inoculation/76 days of gestation)
Long-term outcome (69 days post inoculation/114 days of gestation)
Super-inoculation of in utero K2-or mock-inoculated piglets with PCV2 (K39)
-
Evaluation of sows–All three sows remained clinically healthy during the study period. The PCV2-specific IPMA Ab titre was 40, 960 in both pre-and post-sera of the sows. PRRSV-specific IPMA and PPV-specific HI Ab titres were < 10 and ranged from < 8 to 8, respectively, in pre-serum, and identical in post-serum.
Macroscopic lesions–At 21 dpi, K2-inoculated foetuses had a normal external appearance (Figure 1A). No gross lesions were observed in the different organs, except for enlarged spleen in one foetus (S2R3). K39-inoculated foetuses showed oedema, hydrothorax, hydroperitonium, ascites and distended abdomens (Figure 1B).
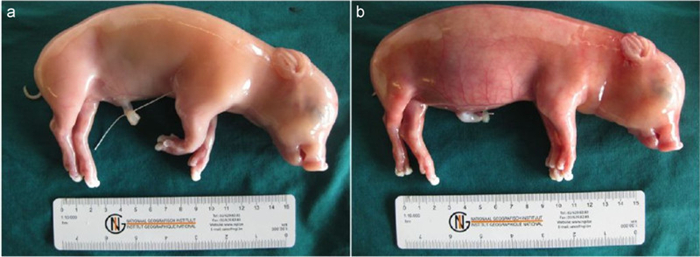
Figure 1. Effects of PCV2 replication on 55-day-old fetuses inoculated collected at 21 days post-inoculation. a) K2-inoculated foetus with normal external appearance. b) Subcutaneous oedema and abdominal distension in K39-inoculated foetus.
Microscopic lesions–No histopathological lesions were observed in the different organs of K2-inoculated foetuses, except one, which displayed infiltration of neutrophils in spleen (S2R3). Histopathological lesions, mainly haemorrhage and infiltration of inflammatory cells, were present in heart, lung, liver, spleen and kidney of K39-inoculated fetuses.
PCV2 replication–In K2-and K39-inoculated foetuses, highest PCV2 titres (log10 TCID50/g) were found in the heart (5.3 ± 1.2 and 5.9 ± 0.1, respectively). Virus titres in different organs are shown in Table 1.
Moderate to high numbers of PCV2-infected cells/10 mm2 tissue (900, 976 and 580 in K2-and 4027, 4350 and 3550 in K39-inoculated foetuses) were estimated in the heart. PCV2-positive cell numbers in different organs are presented in Table 1.
Serology–Both K2-and K39-inoculated foetuses were negative ( < 10) for PCV2-specific IPMA Ab, except one (inoculated with K39), which developed very low anti-PCV2 titres (40).
-
Evaluation of sows–All four sows remained clinically healthy during the study period. The PCV2-specific IPMA antibody titre was 40, 960 in both pre-and post-sera of the sows. PRRSV-specific IPMA and PPV-specific HI antibody titres were < 10 and ≤8, respectively, in pre-serum, and identical in post-serum.
Evaluation of piglets at birth–Intra-foetal inoculation of six foetuses each from two sows with K2-high dose-104.3 TCID50 at 45 days of gestation resulted in five mummies and one live-born piglet in both cases. Similar results were obtained with six foetuses each from the other two sows inoculated with K2-low dose-102.3 TCID50 (Table 2). Recovered mummies had variable crown-rump lengths, indicating that foetuses died at different times in utero (data not shown). Live-born in utero K2-and mock-inoculated piglets appeared clinically normal at birth.
Virus titration in mummies–PCV2 virus was recovered from all mummies with variable titres of 103.7-5.7 TCID50/g tissue.
PCV2-PCR of serum samples collected at birth–Liveborn piglets, including two K2-inoculated piglets, were negative for PCV2 DNA.
Serology–Live-born K2-and mock-inoculated piglets were negative ( < 10) for PCV2-specific IPMA Abs at birth (Table 3).
Pig no. Prenatal life Prenatal life Intra-uterine inoculation At 45 days of gestation Inoculated dose of K2 Oronasal inoculation at 6 or 8 days after birth PCV2 replication IPMA antibody titres 14 dpsi 21 dpsi 28 dpsi at birth 0 dpsi 7 dpsi 14 dpsi 21 dpsi 28 dpsi ILN ILN ILN VT IIF VT IIF VT IIF K2 K39 K2 K39 K2 K39 K2 K39 K2 K39 K2 K39 S4F1 K2 104.3 K39 < 1.7 2 2.3 0 2.3 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 10 160 160 40 S4F4 mock K39 < 1.7 0 < 1.7 0 3.3 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 160 640 160 10240 S4F5 mock K39 < 1.7 0 2.3 0 2.3 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 640 2560 640 10240 S4F6 mock K39 < 1.7 0 2.3 0 2.3 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 160 2560 640 10240 S5F4 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 S5F5 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 S5F6 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 S6F1 K2 102.3 K39 5.6 76 5.1 86 1.9 8 < 10 < 10 640 640 640 640 640 640 10240 2560 40960 10240 S6F4 mock K39 < 1.7 0 3.0 2 2.3 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 160 2560 640 10240 S6F5 mock K39 < 1.7 0 2.0 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 40 40 640 2560 2560 40960 S6F6 mock K39 < 1.7 0 2.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 160 2560 2560 10240 S7F4 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 S7F5 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 S7F6 mock mock < 1.7 0 < 1.7 0 < 1.7 0 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 < 10 IPMA = Immunoperoxidase monolayer assay. PCV2-specifi c antibody titres in pig serum during postnatal life were determined using IPMA; dpsi = days post super-inoculation; ILN = inguinal lymph node; VT = Virus titres (log10 TCID50/g); IIF = Indirect immunofl uorescence staining (number of PCV2-positive cells/10 mm2 ); Immunostimulated pigs are shown in bold. Table 3. PCV2 replication and IPMA antibody titres in pigs inoculated with PCV2-K2 (or mock-inoculated) during prenatal life (at 45 days of gestation) followed by oronasal inoculation with PCV2-K39 (or mock-inoculation) during postnatal life
-
Evaluation of piglets–All piglets (two K2-K39, six mock-K39 and six mock-mock inoculated) were clinically healthy during the study period. No disease was observed.
PCV2 replication (high-dose K2 in utero)–PCV2 titres in ILNs (21 and 28 dpsi) varied from 2.3 to 3.3 in mock-K39 piglets (S4F4-S4F6). Low-level PCV2 replication (up to 2.3) in ILNs of S4F1 (K2-K39) was observed. PCV2-positive cells were detected only in ILNs of S4F1 (14 dpsi) (Table 3), and identified as K39+ K2- .
PCV2 replication (low-dose K2 in utero)–PCV2 titres in ILNs (S6F4-S6F6) at 21 and 28 dpsi varied from 2.0 to 3.0. One of the piglets (S6F1) produced very high viral titres in ILNs at 14 dpsi (5.6) and 21 dpsi (5.1), which were significantly reduced at 28 dpsi (1.9) (Table 3). Among the mock-K39-inoculated piglets, only S6F4 had two PCV2-positive cells in ILN (21 dpsi). In S6F1 (K2-K39), moderate to high numbers of PCV2-positive cells/10 mm2 were detected in ILNs (8 to 86 positive cells/10 mm2 ) (Table 3) and double immunofl uorescence staining further confirmed the cells as K2+ K39- .
Serology–Super-inoculation of piglets with K39 during postnatal life resulted in seroconversion against PCV2 at 21 dpsi, except in S6F1 and S6F5, which seroconverted at 0 dpsi and 14 dpsi, respectively. Highest titres of IPMA Abs were detected at 28 dpsi in all PCV2-inoculated piglets. In S4F1 (K2-K39), Ab titres were higher against K39 than K2. Different results were obtained with the other K2-K39 piglet (S6F1), which had already developed PCV2-specific Abs at 0 dpsi. In this case, at 21 and 28 dpsi, anti-K2 titres were higher than anti-K39 titres (Table 3). None of the piglets developed neutralising Abs against K2 or K39, except S6F1 (K2-K39), which developed neutralising Abs against K2 at 28 dpsi (data not shown).
Short-term outcome
Long-term outcome
Super-inoculation of in utero K2-or mockinoculated piglets with PCV2 (K39)
-
At 21 dpi, K39 induced pathological lesions, validating earlier descriptions of PCV2 infection in porcine foetuses which caused pathology in internal organs suggestive of heart failure (Saha D, 2010). K2 did not induce pathology in mid-gestational foetuses despite higher titres than K39. One possibility to explain this finding is that K2 requires more time ( > 3 weeks) to induce pathology in foetuses. Despite similar PCV2 titres in hearts of K2-and K39-inoculated foetuses, K2-positive cells were 4.9 times lower than K39-positive cells. This may be due to the small focal distribution of K2 infection, compared to wide dispersion of K39 infection throughout heart tissue. Although K2-positive cell levels were lower, the number of apoptotic cells in hearts of K2-and K39-inoculated foetuses was similar (data not shown).
While K2 did not induce pathology in mid-gestational foetuses, intra-foetal inoculation of a high or low dose of K2 at 45 days of gestation resulted in a negative longterm effect (10 mummies out of 12 K2-inoculated foetuses), confirming the hypothesis that K2 needs longer time to cause foetal damage. In the two in utero K2-inoculated live-born piglets, K2 virus and specific antibodies were not detected at birth, indicating that young foetuses had fully controlled K2 infection, possibly through raising an innate immune response. Most cellular components of innate immunity, including natural killer (NK) cells, are found in porcine foetuses before 45 days of gestation (Sinkora M, 2003) but do not show killing activity until activation by microbial pathogens (Sinkora M, 2009). It is possible that NK cells gain their full functional status upon intra-foetal inoculation of PCV2 at 45 days of gestation, which eventually restricts PCV2 replication in foetuses. Wrong inoculations can be excluded, since we have demonstrated 100% success in performing transuterine, intra-foetal inoculations in the past (Saha D, 2010; Saha D, 2011). The accuracy of intra-foetal inoculation of K2 is further confirmed by recovery of viruses from the mummies.
Postnatal super-inoculation of in utero mock-inoculated piglets with K39 resulted in low levels of K39 replication, clearly signifys that K39 has different replication kinetics from foetal to early postnatal life. This finding confirms the results of Sanchez RE (2003). Super-inoculation of in utero K2-inoculated piglet (S4F1) with K39 did not result in disease, consistent with the observed low level of viral replication. High PCV2 replication is a prerequisite for the successful development of disease or PMWS (Meerts P, 2006). The late onset of humoral immune response in S4F1 indicates somewhat disturbed immunological response. Serological analysis indicated that viral replication in S4F1 was associated with K39, not K2, in view of the higher anti-K39 Ab levels. Although viral replication was associated with K39 in both K2-K39 (S4F1) and mock-K39 (S4F4 to S4F6) piglets, the S4F1 piglet had exceptionally low anti-K39 titres, compared to mock-K39 piglets. A low anti-K39 titre in S4F1 may indicate a state of partial immunotolerance, which develops due to intra-foetal K2 inoculation at 45 days of gestation. Immunofl uorescence staining similarly showed that viral replication in S4F1 was associated with K39, not K2, which was further established by sequencing of the capsid gene (data not shown). Our results clearly indicate that K2 infection is fully controlled by the young foetus without full priming of the immune response. S6F1, inoculated with a low dose of K2 in utero, developed a PCV2-specific immune response early after birth, indicative of re-activation of K2. This may be associated with parvovirus vaccination after birth. Super-inoculation of S6F1 with K39 led to extremely high viral replication, which was subsequently remarkably reduced, probably due to the appearance of K2-neutralising antibodies. Serological analysis showed higher levels of anti-K2 IPMA Abs than anti-K39 Abs, signifying that viral replication in S6F1 is associated with K2, not K39. K2 replication was further confirmed by immunofluorescence staining and sequencing of the capsid gene (data not shown). Based on the results, we suggest that K2 infection is silent during foetal life but activated after birth upon vaccination. Another possibility is that K39 replication occurs in S6F1, but at a very low level in the period before sampling of the lymph nodes (14 dpsi).
None of the piglets (except S6F1 at 28 dpsi) developed neutralising Abs. The appearance of neutralising antibodies against PCV2 could be age-dependent. An earlier study by Meerts and co-workers (2006) showed that young pigs, especially CDCD or specific pathogen free (SPF) piglets, developed no or low-level neutralising antibodies against PCV2. Cytotoxic T lymphocytes (CTL) are thought to play a role in determining the immune responses capable of neutralising PCV2.
In conclusion, our study demonstrated that PCV2 K2 is not pathogenic to porcine foetuses over a short-term period, while K39 displays pathogenicity. However, longterm infection with K2 resulted in the birth of both mummies and viable piglets. Moreover, super-inoculation of live-born K2-infected piglets with K39 did not result in disease. Further studies are necessary to identify PCV2 strains with very low pathogenicity to inoculate foetuses with the aim of producing PCV2-immunotolerant pigs. Work in this context is ongoing.
-
We are grateful to Dr. Allan and Dr. McNeilly for providing F190 and F217 antibodies. We additionally thank Zeger, Bart, F. Backer, Sjouke, Lennert, Dominique, Leslie, Annebel, Kalina, Ilias, Eva, Jun, Liping, Amy, Gert, Hossein, Carine, Lieve, Chantal, Melanie, Nele, Irene, Ine and Ytse for help during surgeries and laboratory analyses of the samples. Dipongkor Saha was supported by a BOF scholarship from Ghent University.
-
All authors declare no competing interests. The animal experiments described in this manuscript were authorised and supervised by the Ethical and Animal Welfare Committee of the Faculty of Veterinary Medicine of Ghent University, Belgium.
-
DS and HJN designed the study. DS, HJN, UUK, MG and MV performed the experiments. DS, HJN, DJL and PM analysed data and wrote the manuscript. RD analysed histopathological data. JVD and LH carried out PCR and sequencing.













 DownLoad:
DownLoad: