-
Hepatitis B virus (HBV) infection causes a wide spectrum of liver diseases in more than 240 million people worldwide (Ott et al. 2012). Patients with chronic hepatitis B (CHB) may progress to HBV-related end-stage liver diseases, such as hepatic failure, cirrhosis or hepatocellular carcinoma (HCC) (Tang et al. 2018). Many international clinical practice guidelines for CHB treatment have suggested the functional cure as an ideal goal for antiviral therapy (Sarin et al. 2016; European Association for the Study of the Liver 2017; Terrault et al. 2018). Functional cure means sustained, undetectable HBV surface antigen (HBsAg) and HBV DNA in serum with or without seroconversion to hepatitis B surface antibody following a finite or indefinite course of treatment, which implies no virological relapse or progression of liver disease after treatment cessation (Lok et al. 2017). Generally, HBsAg loss is considered as a satisfactory biomarker for functional cure. However, approved antiviral agents including nucleotide analogues and interferons, fail to achieve HBsAg clearance in the majority of CHB patients. Therefore, it is urgent to identify new targets for therapeutic intervention and develop innovative drugs for curing CHB.
There are two major obstacles to HBV clearance in CHB patients, including the persistence of HBV covalently closed circular DNA (cccDNA) and the defective HBV specific T cell response (Yuen et al. 2018). Since the cccDNA acts as the authentic template for viral replication and gene expression, the difficulty in clearing cccDNA microchromosomes from the nucleus of hepatocytes is an important reason for the relapse after stopping treatment. HBV-specific T lymphocytes are the most important effector cells for the clearance of HBV infection. They directly remove cccDNA or other viral components by granzyme and perforin-induced cytotoxic pathway to induce hepatocyte apoptosis. Moreover, they can secrete antiviral cytokines such as IFN-γ and TNF-α, to inhibit viral replication and induce cccDNA degradation in hepatocytes non-cytopathically (Guidotti et al. 2015). However, HBV-specific T cells are characterized by multiple quantitative and functional defects in CHB patients (Yuen et al. 2018). Therefore, compared to direct antiviral agents, immune modulation strategies for enhancing the function of HBV-specific T cells can not only control HBV replication and reduce HBV antigen levels, but also specifically eliminate the persistence of cccDNA by inducing hepatocytes apoptosis.
Cellular inhibitors of apoptosis proteins (cIAPs) are a family of highly conserved endogenous anti-apoptotic molecules that inhibit caspase activity. It is now clear that cIAPs promote cell survival via a much broader spectrum of action than just by caspase regulation. Recent findings suggest that the cIAPs are involved in the regulation of innate and adaptive immunity, a function attributed to their E3 ubiquitin-ligase activities (Beug et al. 2012). cIAPs participated in multiple pathogen-recognition receptors signaling pathways and were necessary for the production of proinflammatory cytokines (Estornes and Bertrand 2015). Moreover, cIAPs antagonists were able to activate non-canonical NF-κB pathway to promote anti-tumor T cell immune response, thereby inhibiting tumor growth or eliminating tumor cells completely (Chesi et al. 2016).
The immune dysfunction is highly similar in tumor development and chronic viral diseases. Interestingly, cIAPs antagonists also had therapeutic efficacy in the treatment of chronic HBV infection. Previous studies in immune-competent HBV mice models have shown that cIAPs prevent TNF-mediated killing of infected hepatocytes and result in HBV persistence. Liver-specific cIAP1 and total cIAP2 deficiency, or cIAPs antagonist Birinapant treatment led to HBV clearance by promoting death of infected cells (Ebert et al., 2015a, b). Based on these observations, the TetraLogic Pharmaceuticals Corporation launched a Phase Ⅰ safety and tolerability study of Birinapant in CHB patients (NCT number: NCT02288208). This study evaluated the addition of Birinapant to CHB patients who were currently receiving tenofovir or entecavir anti-viral therapy. Unfortunately, this clinical study was terminated in February 2016 due to cranial nerve palsies observed in patients.
APG-1387 is a novel Smac mimetic and highly specific antagonist for cIAPs, which is independently developed by Ascentage Pharma in China. It degrades cIAPs by mimicking the endogenous Smac molecule to induce programmed cell death or apoptosis (Li et al. 2016). The anti-tumor activities of APG-1387 have been well demonstrated in a panel of cancer cell lines and xenograft tumor models (Li et al. 2018; Pan et al. 2018). APG-1387 could induce apoptosis of PLC/ PRF/5 which was HBV positive both in vitro and in vivo. Moreover, APG-1387 was found to promote NK cell proliferation and to enhance T cells function by reducing Treg differentiation, down-regulating PD1 expression and inducing TNF-α and IFN-γ secretion in CD4 and CD8 T cells (Pan et al. 2018). Recently, Phase Ⅰ dose escalation clinical studies with APG-1387 in solid cancers were completed in China and Australia. The preliminary results showed excellent pharmacokinetic and pharmacodynamic profile and no drug related safety issues (Xu et al. 2018).
To clarify whether APG-1387 could be used for HBV therapy, we firstly examined the direct antiviral effect of APG-1387 on HBV replication in HepG2.2.15 cells. The results showed that APG-1387, as well as Birinapant, could not inhibit HBV but slightly enhanced HBV replication at lower concentration (< 10 nmol/L). This observation was consistent with a previous study, which showed that over expression of cIAP2 in hepatocytes could inhibit HBV replication by accelerating the ubiquitin–proteasomemediated destruction of polymerase (Wang et al. 2011). Further, our research group examined the antiviral activity in chronic HBV infection mouse models and explored the underlying mechanism. APG-1387 showed strong antiviral activity and effectively eliminated HBsAg and viral DNA in HBV persistent animals, with single agent and weekly dosing. It degraded liver cIAPs to sensitize the HBV infected hepatocytes to immune-mediated cell killing, thus promoting HBV-specific T cells-mediated clearance of DNA and antigens. The potential advantage of cIAPs inhibitors in the treatment of HBV infection, is their ability to preferentially eliminate infected cells without affecting healthy cells, which is largely dependent on the virus specific T cells recognition. Compared with Birinapant, although the mode of action was similar, APG-1387 exhibited superior efficacy and safety in animal experiments.
Based on above results, the China Food and Drug Administration (CFDA) has accepted the Investigational New Drug (IND) application of APG-1387 for the treatment of HBV infection in November 2017. Currently, a Phase Ⅰ Study of the Safety, Pharmacokinetic and Pharmacodynamic Properties of APG-1387 in CHB patients has been started in Nanfang Hospital, Southern Medical University (NCT number: NCT03585322). This study is a multi-center, single-agent, open-label, Phase Ⅰ doseescalation study and consists of four dosing schedules follewed by escalation after confirming safety. A total of 24 CHB patients without antiviral treatment such as nucleotide analogues and interferons within 6 months before screening will be participated in the study. APG1387 will be administrated via intravenous infusion, once a week for consecutive 4 weeks as one cycle. Initially, the start dose is 7 mg, and will be increased in subsequent cohorts, to 12 mg, 20 mg, 30 mg, and 45 mg accordingly. Three patients in 7 mg and 12 mg cohorts and six patients in 20 mg, 30 mg, and 45 mg cohorts will be recruited. The detailed protocol about the treatment and follow up information is presented in Fig. 1.
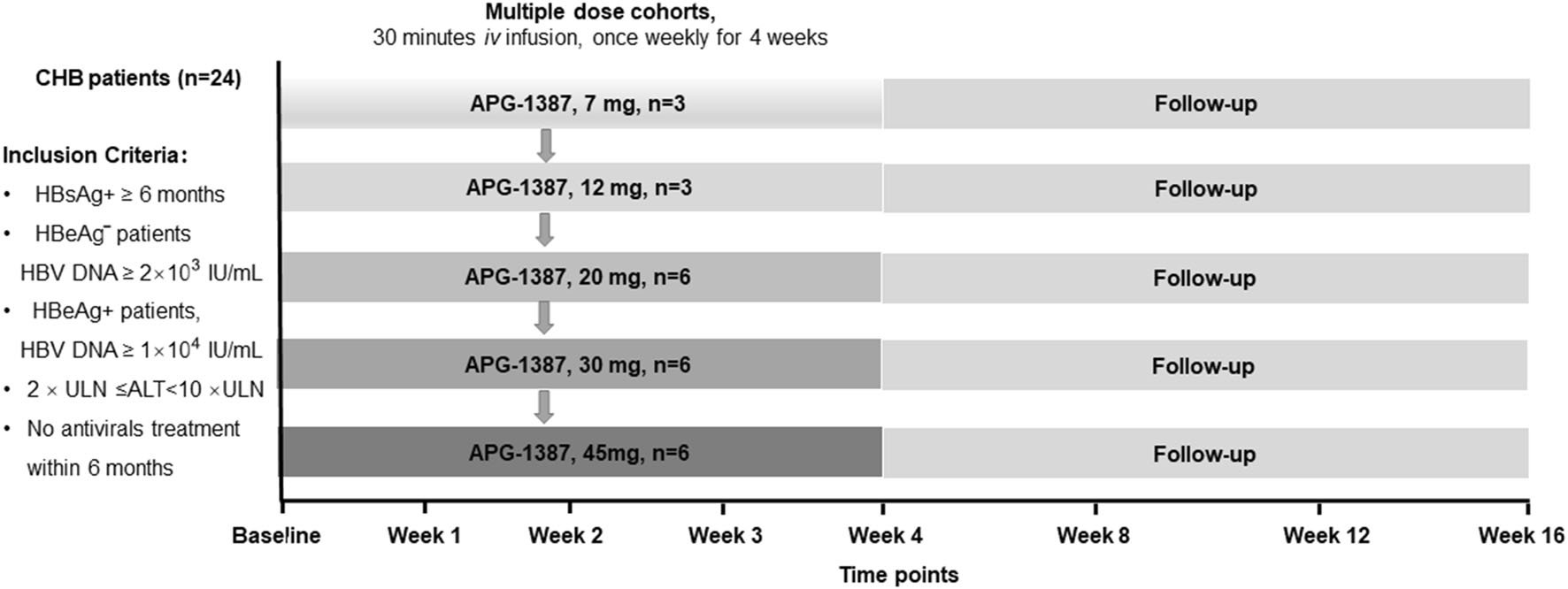
Figure 1. A phase Ⅰ study of the safety, pharmacokinetic and pharmacodynamic properties of APG-1387 in patients with chronic hepatitis B. ULN upper limits of normal, ALT alanine aminotransferase.
Until now, how to achieve functional cure in CHB patients remains a great challenge in scientific and clinical research (Block et al. 2018). With a unique apoptosis and immunoregulation mechanism, APG-1387 has the potential to clear HBV infection in patients and sheds light on HBV cure research. However, we must pay great attention to this therapeutic strategy designed to boost host immunity and induce hepatocyte apoptosis, as it carries the inherent risk of inducing liver damage or other side effects. In the future, further exploration of the immunopathogenesis of CHB will be helpful to propose new strategies for curing hepatitis B.
HTML
-
This work was partly supported by Grants from the National Natural Science Foundation of China (81641173) and the Collaboration and Innovation Health Care Major Project of Guangzhou (201604020010).
-
The authors declare that they have no conflict of interest.
-
The authors declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: