HTML
-
Suitable human immunodeficiency virus type 1 (HIV-1) infected animal models assist the elucidation of viral pathogenesis and the evaluation of antiviral strategies. Currently, several models based on nonhuman primates (NHP) or humanized mice are available (Kumar et al. 2016). These NHPs, including Northern pig-tailed macaque (Macaca nemestrina) and Indian Rhesus monkey (Macaca mulatta), have been used to establish the acutely and persistently viral infection models by infection with simian immunodeficiency virus (SIV) or chimeric simian/human immunodeficiency virus (SHIV). These models are useful for studying viral transmission, pathogenesis, drugs, antibodies and candidate vaccines (Dinoso et al. 2009; Kline et al. 2013; Hessell and Haigwood 2015). Meanwhile, these models have several noted limitations, such as the inability to precisely examine human specific immune functions because of genetic differences in major histocompatibility complex (MHC) genes, the difficulty to operate sufficient sample size due to their expensive costs, and the mounting ethical concerns of experimentation with large animals (Evans and Silvestri 2013). To avoid the problems encountered in these NHP models, the humanized mouse models offer an alternative. The versatility of inbreeding allows mice to reproduce rapidly and produce strains with a clear genetic background and well defined immune systems (Shultz et al. 2007).
To construct humanized mice, human PBMCs, stem cells, or lymphoid tissues have been used for transplantation into immunodeficient mice (Zhang and Su 2012). Three decades ago, mice with severe combine immune deficiency (SCID) that lacked of both T cells and B cells had been used for the reconstruction of human immune cells or system. SCID mice transplanted with human PBMCs can construct SCID-hu PBL (human peripheral blood leukocytes) mice (Ganick et al. 1980; Mosier et al. 1988), and those transplanted with human fetal liver and thymus can construct SCID-hu Thy/Liv mice (McCune et al. 1988). However, SCID-hu PBL mice lack human lymphatic organs and have been proved to cause graft versus host disease (GVHD), while SCID-hu Thy/Livmice have low frequency of human cells in their blood and peripheral organs.
B-NSG (NOD-PrkdcscidIl2rgtm1/Bcge) mice have Nodscid-il2rg null gene background and a longer lifespan than NOD/SCID mice, and those mice have recently been experimented for humanization (Shultz et al. 1995; Ito et al. 2002). These B-NSG mice display severe immune defects, lacking mature T cells, B cells and functional NK cells due to a defective rearrangement of the T cell receptor and immunoglobulin genes. Therefore, these homozygous mice have been shown to be suitable for the transplantation and growth of human PBMCs and hematopoietic stem cells (HSCs) (Ishikawa et al. 2005; Strowig et al. 2011). The humanized B-NSG mice transplanted with bone marrow/liver/thymus (BLT) can afford differentiation of human HSCs into a variety of human cells, including lymphocytes, NK cells, monocytes, macrophages and dendritic cells, and these human cells can be detected in multiple tissues and organs, including blood, bone marrow, lymph nodes, spleen, thymus, liver, lung, digestive tract and reproductive tract (Lan et al. 2006; Brainard et al. 2009; Chung et al. 2015). Whereas, the B-NSG mice-based Hu-thy/liv model can only allow differentiation of human T cells (Honeycutt et al. 2013; Nixon et al. 2017). These humanized mice have been used for infection with HIV-1 to establish the acute and persist infection models to elucidate the processes of viral invasion, immuno-pathogenesis and to evaluate strategies for HIV-1 prevention, treatment and eradication (Brooks et al. 2003; Sun et al. 2007; Jiang et al. 2008; Brainard et al. 2009; Marsden et al. 2012; Zhang and Su 2012; Gruell and Klein 2017; Nixon et al. 2017). Meanwhile, the limitation of these mice models includes expensive experimental cost, delicate surgical procedures, and months of time required for reconstruction.
In this study, we construct a rapid and convenient humanized mouse model of HIV-1 infection using B-NSG mice which were engrafted with human PBMCs via tail vein injection. We then demonstrated that the humanized mice can be infected by HIV-1, and the viral infection recapitulates the expected changes of T cell subsets in human HIV infection, while cART suppresses viral replication and restores T cell subset abnormalities.
-
NOD-PrkdcscidIl2rgtm1/Bcge (B-NSG) mice were purchased from Beijing Biocytogen and housed in a pathogenfree animal facility at Institut Pasteur of Shanghai. Human peripheral blood buffy coats were purchased from Changhai Hospital, Shanghai, China, and PBMCs were isolated from the buffy coats of healthy donors by Ficoll-density gradient centrifugation as described previously (Li et al. 2017). All procedures were conducted in compliance with a protocol approved by the Institutional Animal Care and Use Committee at Institut Pasteur of Shanghai. All experiments were performed in accordance with relevant guidelines and regulations.
-
5-weeks old female B-NSG mice were transplanted with human healthy PBMCs (5 × 106 cells/mouse) via tail intravenous injection. Mice were humanized with PBMCs from different donors separately. The peripheral blood from the retro-orbital sinus was collected each week after transplantation and the differentiation of human CD45+ cells were monitored by flow cytometry. For HIV-1 infection, humanized mice with 3-weeks human PBMCs transplantation were selected for infection with HIV-1/JRCSF (CCR5-tropism) (10 ng p24Gag/mouse) by intraperitoneal injection. Viral replication in plasma and tissues of humanized mice was quantified by quantitative real-time PCR (qRT-PCR) to detect the production of gag mRNA.
At 4-weeks post-infection (w.p.i.), mice were treated with combination antiretroviral therapy (cART). cART regimens are composed of nucleoside reverse transcriptase inhibitor Tenofovir disoproxil fumarate (TDF; 205 mg/kg), nucleoside reverse transcriptase inhibitor Emtricitabine (FTC; 211 mg/kg) and integrase inhibitor Raltegravir (RAL; 80 mg/kg) (Satheesan et al. 2018). Triple combination drugs were dissolved in 3% DMSO, 40% PEG-400, 2% Tween-80 and 56% ddH2O. Mice were administered daily via intraperitoneal injection.
-
Total cellular mRNAs from plasma and tissues were extracted using the QiaAmp Viral RNA Mini Kit (Qiagen, Germany) and then reverse transcribed into cDNA using the ReverTra Ace qPCR RT Master Mix with gDNA Remover Kit (Toyobo, Japan). qRT-PCR was performed on the ABI 7900HT Real-Time PCR system (Applied Biosystems, United states) (50× ROX and 2× Gold Taq-Man Mixture; CMBIO), with an initial denaturation step at 95 ℃ for 10 min, amplification with 40 cycles of denaturation (95 ℃, 15 s) and annealing (60 ℃, 1 min). HIV-1 gag primers were used: forward primer, 5′-GGT GCG AGA GCG TCA GTA TTA AG-3′, reverse primer 5′-AGC TCC CTG CTT GCC CAT A-3′, and probe, 5′-FAM-AAA ATT CGG TTA AGG CCA GGA GGA AAG AATAMRA-3′ (Ye et al. 2017).
-
Peripheral blood was collected from retro-orbit. Spleen, bone marrow and liver were gained at necropsy to make the single cell suspension. Red blood cells were eliminated by using Red Cell Lysis buffer solution (Multi Sciences, Hangzhou, China). Monoclonal antibodies against these specific antigens of human and mouse cells were listed as follows: APC-mouse CD45 (30-F11, eBioscience, United states), FITC-human CD45 (HI30, eBioscience), PE-human CD3 (UCHT1, eBioscience), percpcy5.5-human CD4 (HIB19, eBioscience), Brilliant Violet 421-human CD8 (RPA-T8, BD Biosciences), APC-cy7-human CCR5(2D7, eBioscience), APC-human CXCR4 (12G5; BD Pharmingen, United states). The stained cells were detected using a Fortessa flow cytometer (BD Pharmingen) and analyzed with FlowJo 7.6.1. software (United states).
-
This software of GraphPad Prism version 7.0. (San Diego, California, United states) was used to perform paired t tests to analyze statistically significant differences.
Ethics Statement
Construction of Humanized Mice, HIV-1 Infection and Antiretroviral Treatment
HIV-1 Replication Assay by qRT-PCR
Flow Cytometry
Statistical Analysis
-
Five weeks old female B-NSG mice were transplanted with healthy human PBMCs via tail vein injection (Fig. 1A). The human CD45+ (hCD45+) leucocytes in mouse peripheral blood were monitored by flow cytometry over time. At 4–7 weeks post transplantation, the number of hCD45+ leucocytes reached a peak, and then declined but remained detectable for an additional 4–5 weeks (Fig. 1B). As shown in a representative mouse, hCD45+ cell population in peripheral blood compromises of both CD4+ and CD8+ T cell subsets. Among the 82.4% of hCD45+ cells, the majority were CD3+ T cells (93.8%), of which, CD4+ and CD8+ T-lymphocytes account for 50.2% and 41.8%, respectively (Fig. 1C). The reconstruction of human B cells and myeloid cells was not observed (data not shown). Taken together, these data show the successful reconstruction of human T-lymphocyte subsets in the peripheral blood of B-NSG mice transplanted with human PBMCs.
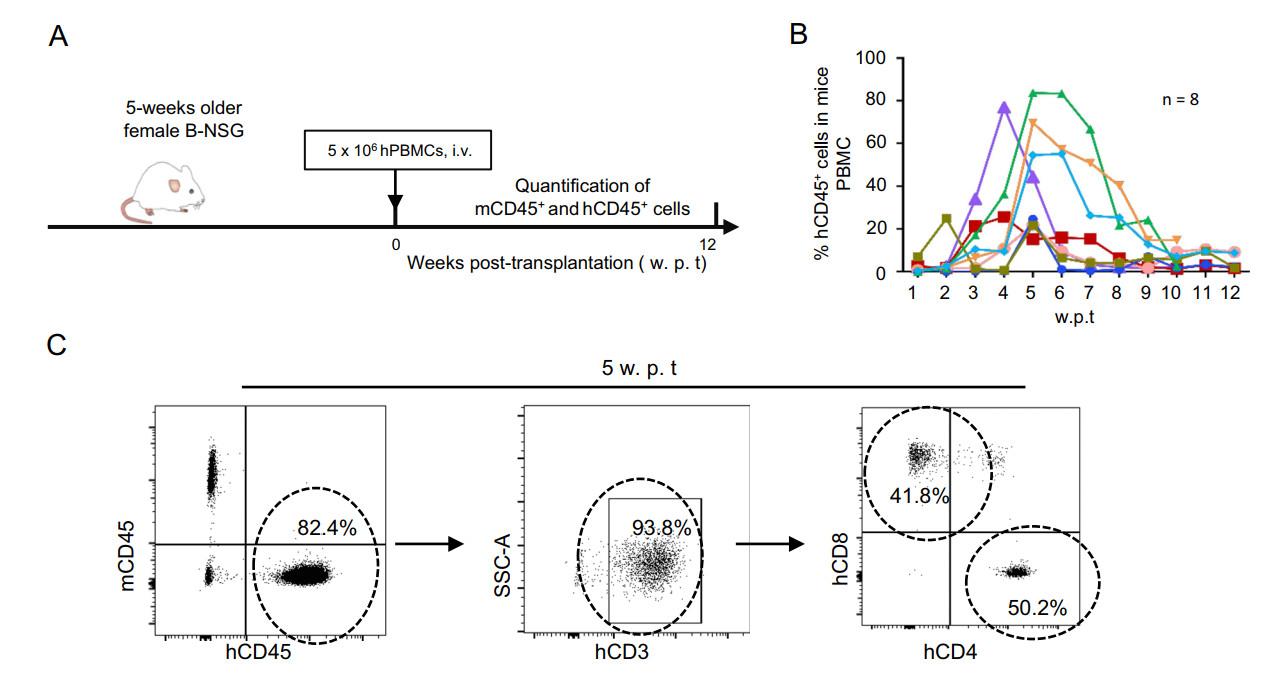
Figure 1. Establishment of humanized B-NSG mice. A Schematic diagram showing the construction of humanized B-NSG mice. 5-weeks old female B-NSG mice were transplanted with human healthy PBMCs (5 × 106 cells/mouse) via tail intraven ous injection, peripheral blood cells were harvested each week after transplantation for detecting mCD45+ and hCD45+ cells with flow cytometry using specific antibodies. B The dynamic of hCD45+ cell population in peripheral blood of humanized mice. Each mouse was humanized with PBMCs from different donors separately, and eight representative mice were shown (n = 8). C The reconstruction of human T-lymphocyte subsets. Peripheral blood cells from one representative mouse with 5-week transplantation were collected, immunostainings were performed using specific antibodies and detected by flow cytometry. SSC-A, side-scattered light area.
-
Next, we examined the susceptibility of these humanized mice for HIV-1 infection. These B-NSG mice with reconstruction of human T-lymphocyte were intraperitoneally (i.p.) injected with HIV-1 (Fig. 2A). Because these reconstructed hCD4+ T cells expressed HIV-1 co-receptor CCR5 (10%–33%), but not CXCR4 (less than 1%) (Fig. 2B), the CCR5-tropic virus HIV-1/JR-CSF was used for infection (Fig. 2A). Viral replication in plasma was monitored longitudinally by qRT-PCR detection for gag gene expression. Results showed a rapid viral replication during the first-week of infection, and viremia reached a steady peak level for the next few weeks (Fig. 2C). The adminstration of a cocktail of TDF/FTC/RAL at 3 w.p.i. markedly suppressed viral replication and reduced the plasma viral load to undetectable level within 1 week of treatment (Fig. 2C). Neither infection nor cART-treatment obviously affected mouse body weight (Fig. 2D). Taken together, these data demonstrate that humanized B-NSG mice can be successfully infected by HIV-1 and viral replication can be suppressed by cART-treatment, and thereby can be used as an in vivo model for studying the establishment of HIV infection and antiretroviral treatment outcome.
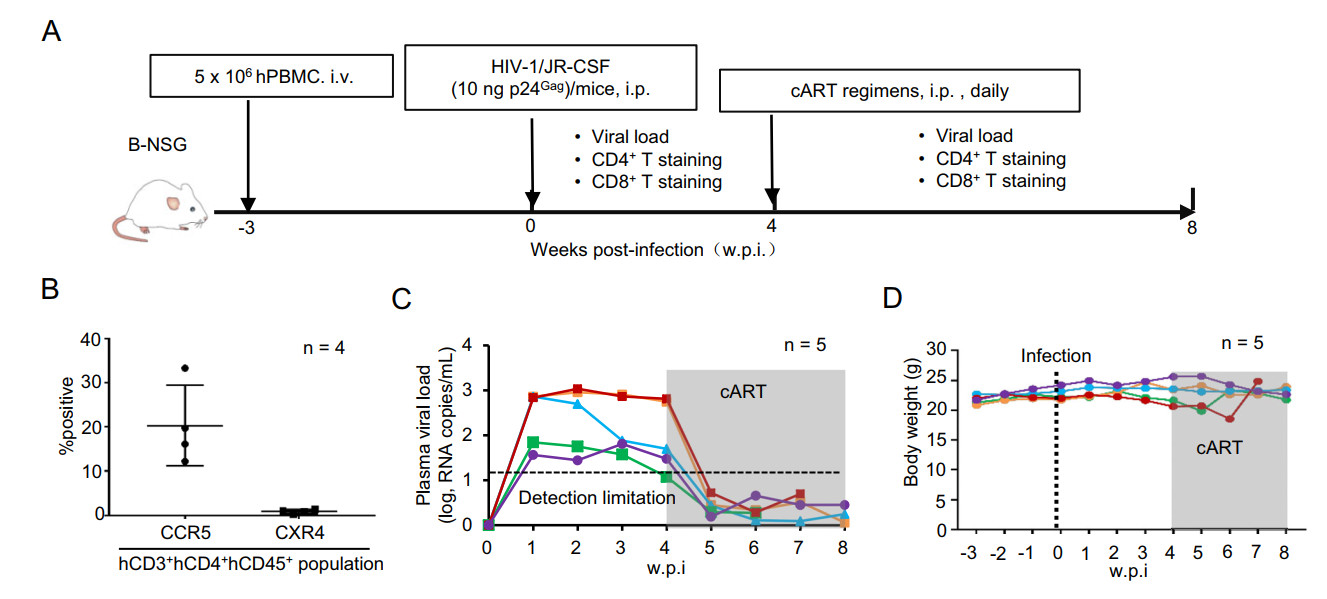
Figure 2. HIV-1 infection of humanized B-NSG mice and cART treatments. A Schematic illustration of HIV-1 infection of humanized B-NSG mice and cART treatments. B-NSG mice with 3-week transplantation were i.p. infected with HIV-1/JR-CSF (10 ng p24Gag/mouse), and 4 w.p.i, cART regimens (TDF/FTC/RAL) was administered daily by i.p. injection and lasted overtime of surveillance. Viral load and cell subsets from retro-orbital blood sampling were monitored. B Assay of HIV-1 co-receptor expression. Cells were immunostained with specific antibodies and detected with flow cytometry, and the expressions of CXCR4 and CCR5 in hCD3+ hCD4+ hCD45+ population were calculated. C Viral load in plasma of humanized mice was quantified by real-time (RT-) PCR to detect the production of gag mRNA. The shaded area indicates cART-treatment. D Mice weights were monitored.
-
HIV-1 infection of human individuals can induce a rapid CD4+ T cell decline and a reverse of CD4+/CD8+ T cell ratio (Lu et al. 2015), and the cART treatment in most cases can normalize these changes (McBride and Striker 2017). Therefore, we next investigated whether the HIV-1 infected-humanized mice could be used to feature these changes. Results from one representative mouse showed that, after being transplanted with human PBMCs for 3 weeks and then infected with HIV-1/JR-CSF for an additional 4 weeks, hCD4+ T cell frequency reduced from 78.5% (before infection) to 12.1% (after infection), and hCD8+ T cell frequency increased from 19.6% to 80.4%. cART-treatment for an additional 3-weeks recovered hCD4+ T cell frequency to 45.9% and reduced hCD8+ T cell frequency to 38.5% (Fig. 3A). Similar results were obtained from four HIV-1 infected-humanized mice (Fig. 3B). HIV-1 infection reversed blood hCD4+/CD8+ ratio in mice, whereas cART-treatment normalized the ratio (Fig. 3C). The dynamic changes of hCD4+ and hCD8+ T cells over time of infection and treatments were monitored (Fig. 3D). HIV-1 infection induced a typically rapid decline of hCD4+ T cells and a compensatory increase of hCD8+ T cells, and the cART-treatment normalized these parameters (Fig. 3D). To sum up, these data demonstrate that HIV-1 infection of humanized mice can recapitulate typical CD4+ T cell and CD8+ T changes observed in human infections, and such abnormality of T cell dynamics can be reversed by cART-treatment, suggesting such a model could be used to examine HIV-1 pathogenesis in vivo.
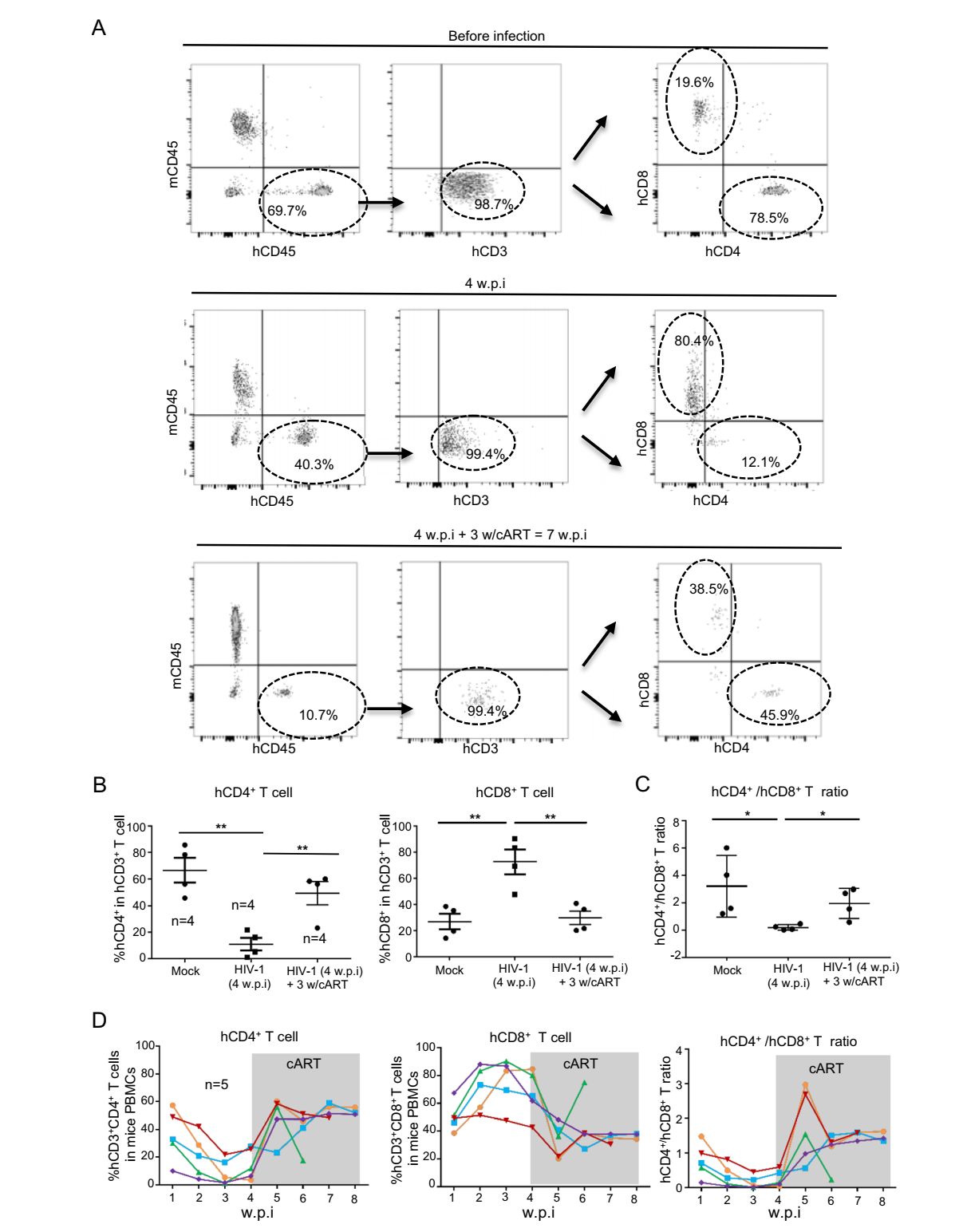
Figure 3. The dynamic of human T-lymphocytes in HIV-1 infectedhumanized mice. A The longitude detection of hCD4+ and hCD8+ T cell. The peripheral blood cells from one representative mouse were harvested at the different times and further analyzed with flow cytometry. B, C The frequencies of hCD4+ and hCD8+ T cell from four humanized mice were summarized (B) and the hCD4+/CD8+ T cell ratio was calculated (C). D The dynamic changes of hCD4+ and hCD8+ T cells over time of infection and cART-treatment. The shaded area indicates cART-treatment. Data are presented as mean ± standard deviation. *P < 0.05 and **P < 0.01 are considered as significant differences in paired t test.
-
Having demonstrated the recapitulation of T cell dynamic features of HIV infection in human peripheral blood, we went on to investigate whether the B-NSG mice can also be used to outline major features of human HIV-1 infection in tissues and whether primary infection starts and latent reservoir virus may form. The new experimental procedures include harvesting tissue samples in addition to the transplantation, infection, cART treatment steps as performed in previous experiments (Fig. 4A). About 2% of hCD45+ cell subset was observed in liver, whereas 20%– 80% of hCD45+ cell population were observed in spleen and bone marrow (BM) after PBMC transplantation, and these numbers were not altered significantly by HIV-1 infection and cART-treatment (Fig. 4B). By quantifying viral RNA copies at 4 w.p.i., HIV-1 replication was observed in spleen and BM, suggesting a successful infection happened; and viral replication could be suppressed to below the level of detection after 3-weeks cART-treatment (Fig. 4C). Accompanied with a successful virological response to treatment, there was limited reconstruction of CD4+ and CD8+ T cell subsets in liver (less than 2% of the whole cells population), but greater reconstruction in spleen and BM. In spleen, CD3+ and CD8+ T subsets displayed 15%–35% and 10% reconstruction, respectively; in BM, CD3+ and CD8+ T subsets displayed 8% and 4% reconstruction, respectively (Fig. 4D). Similar to that being observed in the peripheral blood, HIV-1 infection for 4-weeks reduced CD3+ T cell but increased CD8+ T-cell frequency, and cART-treatment for 3-weeks partially normalized these cell population (Fig. 4D). Taken together, these results demonstrate that the HIV-1 infection-induced changes of human T-lymphocytes can also be recapitulated in several tissues of HIV-1 infected B-NSG mice.
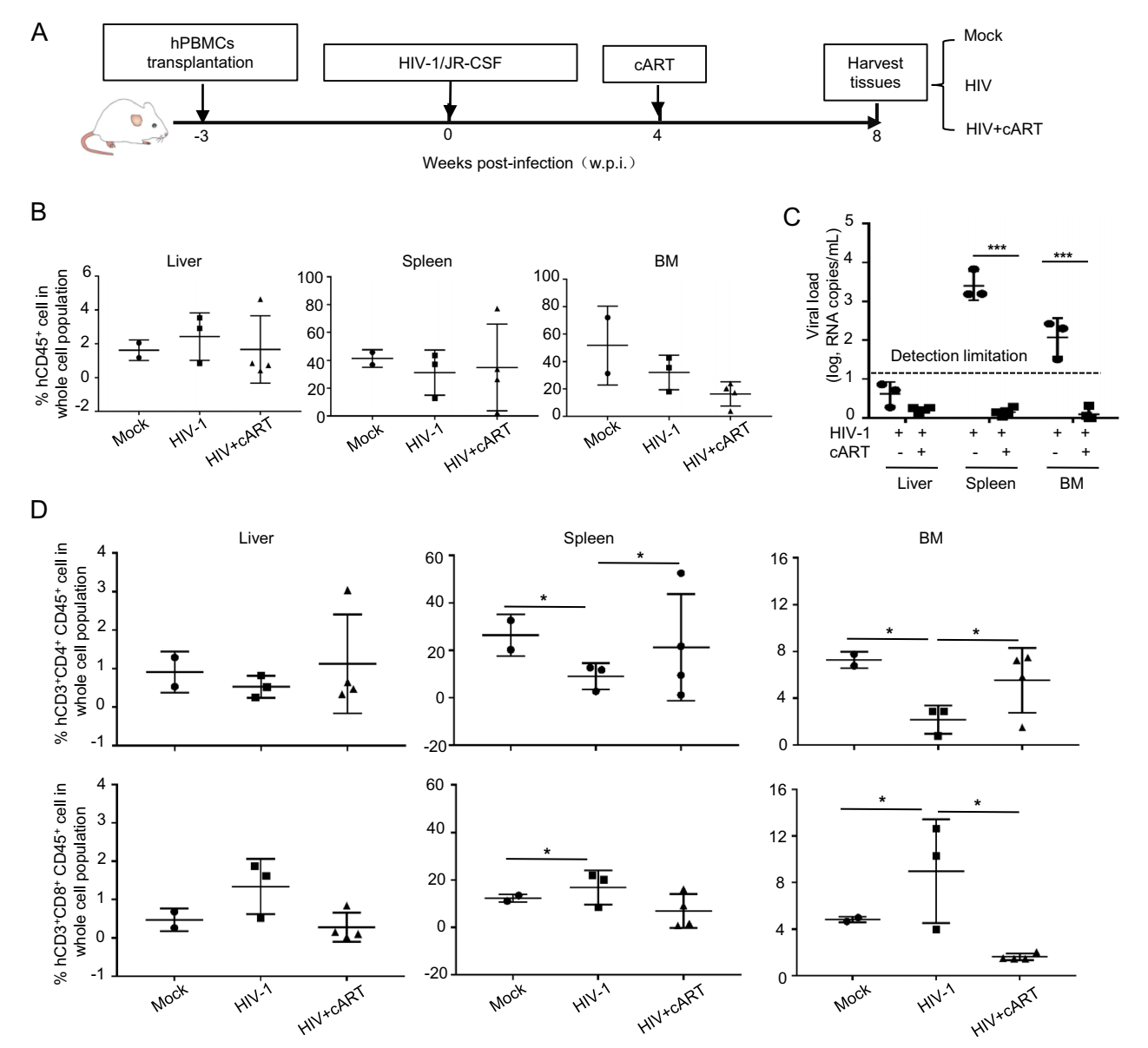
Figure 4. The reconstruction of human T-lymphocytes in tissues of B-NSG mice and viral infection. A Schematic illustration of HIV-1 infection, cART treatment and tissue harvest. B Reconstruction evaluation by detecting hCD45+ cell. Humanized mice were infected by HIV-1 and then treated with cART as above, and the hCD45+ cells in different tissues were detected with flow cytometry. C Viral replication in different tissues was quantified by real-time (RT-) PCR to detect the production of gag mRNA. D The distribution of hCD4+ and hCD8+ T cells in different tissues was evaluated. Data are presented as mean ± standard deviation. *P < 0.05 and ***P < 0.001 are considered as significant differences in paired t test.
Humanization of B-NSG Mice by Transplanting Human PBMCs
Humanized B-NSG Mice Can Be Infected by HIV-1 and Viral Replication Can Be Ceased by cART
HIV-1 Infected-Humanized Mice Feature Similar T-lymphocyte Dynamic Changes Observed in HIV-1 Infected Humans
The Examination of Human T-lymphocyte Changes in Tissues of HIV-1 Infected B-NSG Mice
-
Humanized mouse models have been widely used to investigate human haematopoiesis, innate and adaptive immunity, autoimmunity, infectious diseases, cancer biology and regenerative medicine (Shultz et al. 2007). In the HIV-1 field, the BLT and Hu-thy/liv mice have previously been used to elucidate viral immuno-pathogenesis and to evaluate strategies for HIV-1 prevention and treatment. These humanized mouse models need to be implanted a piece of fetal liver tissue between two pieces of fetal thymus like sandwiches. Although those models have a more complete thymus development process and can generate an adaptive immune response, the technical and traumatic surgery, the acquisition of human tissue and the over a 2-month rebuilding cycle bring many difficulties.
The B-NSG mice used in this study have severe immune defect phenotype and are suitable for the transplantation and growth of human PBMCs and HSCs (Ishikawa et al. 2005; Strowig et al. 2011). In this study, we construct a more convenient model of HIV-1 infection using humanized B-NSG mice. At as early as 3-weeks post-transplantation, these mice showed repopulation of human CD45+ cells in peripheral blood, and these human CD45+ cells were mainly comprised of CD4+ and CD8+ T cells. These reconstructed CD4+ T cells mainly expressed the HIV-1 co-receptor CCR5, and the infection of these humanized mice with CCR5-tropic HIV-1/JR-CSF induced viremia within 1-week. HIV-1 infection induced a rapid CD4+ T cell depletion and a CD8+ T cell increase, which recapitulated the major features of acute HIV-1 infection in humans. Therefore, these HIV-1 infected B-NSG mice - provide a model suitable for studying HIV-1 induced CD4+ T cell decline and evaluating blocking strategies for HIV-1 transmission. Moreover, our data show that the administration of cART suppressed viral replication to an undetected level. Therefore, our mouse model may have potential to use to evaluate HIV-1 latency-reversing agents and strategies for latency eradication. Furthermore, the reconstruction of the human CD4+ and CD8+ T cells in spleen and BM also support the usage of this model for studying viral dynamic and host immune responses in tissues.
In fact, transplanting human PBMCs to construct HIV-1 infected mice models have been reported (Kim et al. 2016; Wu et al. 2016). In the Wu's and Kim's models, the NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice were used, and i.p. injection for engraft was adopted. In our study, the B-NSG (NOD-PrkdcscidIl2rgtm1/Bcgen) mice was used, which are more suitable for the transplantation and growth of human PBMCs and HSCs. Further, we used i.v. injection of PBMCs, instead of their i.p. route. The i.v route has advantages for humanization, by which a significantly higher level of human CD45 cells engraftment can be acquired than that of through i.p. route. The i.v. injection can directly put human PBMCs into the circulation system, whereas for i.p. injection, a period of 7–14 days is required for human PBMCs to drain from the peritoneal cavity into the circulation. Thus, i.v. injection has the ability to engraft higher levels of human PBMCs at lower donor cell doses (5 × 106 cells/mouse used in our study), permitting the better reconstruction; further, i.v. injection permits immediate interaction between the human immune system with the allograft (King et al. 2008). Additionally, for cART-treatment to suppress HIV-1 infection, we used i.p. injection of drugs instead of oral administration in Kim's model, guaranteeing the same dose of drugs per mouse per day.
To suppress HIV-1 infection in our mice model, the cART regimens composed of nucleoside reverse transcriptase inhibitor TDF, nucleoside reverse transcriptase inhibitor FTC and integrase inhibitor RAL were used. The triple combination of TDF/FTC/RAL is currently used for clinical treatment of patients, therefore, to recapitulate patient treatment, this drug-combination has been widely used in the HIV-infected humanized mice models (Honeycutt et al. 2013; Satheesan et al. 2018).
We appreciate that the humanized mouse model also has deficiencies. For example, the engrafted human PBMCs can only differentiate human T cells, not other cells which may affect T cells' functions. A modified strategy that uses human CD34+ hematopoietic stem cell for injection may provide an opportunity to markedly improve the reconstitution of other elements of the human immune system both in tissues and peripheral blood (McDermott et al. 2010; Covassin et al. 2013).
In summary, in this study, we construct a rapid and convenient humanized mouse model for HIV-1 infection using B-NSG mice. The establishment of HIV-1 infected humanized B-NSG mice not only provides a model to study virus invasion-induced T cell dynamic changes but also offers an effective tool for evaluating antiviral strategies.
-
This work was supported by Grants to JHW from the National Grant Program on Key Infectious Disease (2018ZX10301101-003-002), the Natural Science Foundation of China (NSFC, 81572001, 81873965), the key project from Chinese Academy of Sciences (QYZDB-SSW-SMC059), and grant to WWS from NSFC (31800152). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
-
TJF, LS and WWS conceived the experiments, designed the experimental flow and performed the experiments. TJF performed statistical analyses. TJF, WWS and JHW wrote the manuscript. TJF, XGY and XJ reviewed the manuscript. WWS and JHW supervised the project. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interest.
-
All procedures were conducted in compliance with a protocol approved by the Institutional Animal Care and Use Committee at Institut Pasteur of Shanghai. All experiments were performed in accordance with relevant guidelines and regulations.














 DownLoad:
DownLoad: