-
Dear Editor,
Marburg virus (MARV) belongs to the Filoviridae family, along with the related Ebola virus (EBOV). Although MARV is less renowned than EBOV, it causes an equally devastating disease, with clinical symptoms similar to EBOV infection and a remarkably high mortality rate. To date, about 600 MARV cases have been reported globally, with an average mortality rate of around 80% (Brauburger et al. 2012). Due to the severity of disease caused by MARV, as well as its threat to global public health, MARV is classified as a risk group 4 pathogen by WHO, neces-sitating containment measures equivalent to biosafety level 4 (BSL-4). Moreover, MARV is considered a potential agent of bioterrorism (Leffel and Reed 2004) and, since 2015, has been listed as one of ten priority pathogens on WHO's Blueprint of Priority. However, drug development specific for MARV is seldomly reported, although some studies for drugs against EBOV have identified compounds that may also be effective against MARV (Madrid et al. 2013; Bixler et al. 2017; Cross et al. 2018). MARV is a single-stranded negative-sense RNA virus. Its genome encodes seven proteins, of which the glycoprotein (GP) is the only membrane protein and is solely responsible for mediating virus adhesion and entry into host cells (Brauburger et al. 2012). Because GP is one of the key elements determining virus infection, it is therefore the main target of MARV drug, antibody and vaccine research. Importantly, despite the fact that MARV and EBOV belong to the same virus family, the amino acid homology between their glycoproteins is only * 30% (Flyak et al. 2015; Flyak et al. 2016). Thus, drugs against EBOV may not be useful against MARV, and the importance of searching for MARV-specific drugs cannot be neglected.
The requirement for BSL-4 facilities greatly restricts the progress of research into MARV. To address this restric-tion, we previously developed a replication-defective, HIV-based pseudovirus expressing MARV GP, and we demonstrated the utility of this pseudovirus in both high-throughput screening in vitro and in a bioluminescent imaging (BLI) mouse model (Zhang et al. 2017). Impor-tantly, this pseudovirus system can be safely used in BSL-2 laboratories. In the present study, we have leveraged our pseudovirus system to screen a large panel of clinically-approved drugs against MARV. The compound library, which was obtained from the National Institutes for Food and Drug Control in China, contains 767 approved drugs that have each passed strict clinical trials and for which the pharmacokinetics, toxicity and side effects have all been clearly defined. The experimental design of the in vitro screen is illustrated in supplementary Fig. S1A. The details of the experiment were shown in Supplementary Materials and Methods. The experiment was optimized and the quality of the screen was confirmed before the formal testing by assessing a set of commonly used parameters (Wang et al. 2014): signal-to-noise ratio (S/B), Z' factor, and coefficient of variation (CV). The high S/B value (769), a Z' factor value of over 0.5 (0.62), and a very low CV (0.5%) indicate the reliability of this screen (supple-mentary Fig. S1B). Following assay validation, compounds were screened as outlined in supplementary Fig. S1C. Initially, a primary screen of all 767 compounds was per-formed using a single dose of 200 lmol/L. This screen eliminated 476 compounds that had inhibition rates of less than 70%. The remaining 291 compounds, which had inhibition rates greater than 70%, were selected as anti-MARV candidates and subjected to a secondary screen. The secondary screen was performed with 4-point or 8-point serial dilution (1:3, from 200 lmol/L) for hit confirmation and determination of half maximal inhibitory concentration (IC50). Notably, since compounds that inhibit HIV transcription or luciferase activity would also result in decreased luminescence, a control virus consisting of the same HIV backbone but expressing the vesicular stomatitis virus (VSV) envelope glycoprotein (p/HIV/VSVG/Fluc) was used to exclude false positive hits. Eighty-seven compounds were identified to be effective (EC50p/HIV/VSVG/Fluc/EC50p/HIV/MVGP/Fluc > 3) after the secondary screen. A counter screen was then performed to assess compound cytotoxicity, which is another important factor that can lead to false positive results. Accordingly, the 87 com-pounds identified in the secondary screen were evaluated in parallel for their cytotoxicity in HEK293T cells. The cell viability of the 87 compounds was tested with Cell Titer-Glo Luminescent (see Supplementary Materials and Methods for details). Compounds were diluted to 4-point or 8-point serial dilutions (1:3, from 200 lmol/L) to calculate EC50p/HIV/MVGP/Fluc, EC50p/HIV/VSVG/Fluc, and 50% cyto-toxic concentration (CC50) at the same time. The selectivity index (SI) was calculated as the ratio of CC50 and EC50pMARV. Finally, 33 hit compounds that had a SI[3 were considered to be effective in vitro (Table 1), with the two anti-malarial drugs, chloroquine phosphate and amodiaquine hydrochloride, exhibiting the highest SI val-ues. Over one third of the 33 hits were psychotropic sub-stances, including antidepressant drugs, as well as drugs used to treat schizophrenia and drugs used to prevent migraines. Eight compounds were proven antihistamine drugs, while six were known to have anticholinergic activity. Three compounds, including diltiazem hydrochloride, propafenone hydrochloride and amlodipine besylate, were calcium channel or sodium channel regu-lators, which modulate the cardiovascular system. Nons-teroidal antiandrogen flutamide and corticosteroid budesonide were also identified as possible anti-MARV candidates (Table 1 and supplementary Fig. S1D). Inter-estingly, many of the drugs identified against MARV clustered into categories similar to those identified in anti-EBOV screens reported by other groups, including anti-histamines, anticholinergics, estrogen receptor modulators, and drugs that affect the brain, nervous, and cardiovascular systems (Kouznetsova et al. 2014; Bai and Hsu 2018). It is also important to note that, because of the nature of the pseudovirus system, the screens used in this study targeted MARV GP specifically. Thus, the positive hits that were identified likely targeted the attachment or entry step of virus infection, whereas drugs targeting other steps of MARV infection, such as replication or release, could not be identified in this study.
Compound name IC50-MARV(lmol/L) IC50-VSV(lmol/L) CC50(lmol/L) SI = CC50/IC50 VSV/MARV Pharmacological classes Chloroquine phosphate 1.7 53.6 > 200 > 116.1 31.1 Antimalarial Amodiaquine hydrochloride 1.4 19.5 79.3 56.6 13.9 Antimalarial Mianserin hydrochloride 5.4 74.8 > 200 > 37.3 13.9 Antidepressant Diltiazem hydrochloride 6.8 53.6 > 200 > 29.4 7.9 Vasodilator, antiarrhythmic Imipramine hydrochloride 5.3 38.2 152.3 28.6 7.2 Antidepressant Pizotyline 4.4 32.7 99.3 22.4 7.4 Prevent migraine; antidepressant Sertraline hydrochloride 1.6 26.3 33.8 21 16.3 Antidepressant Promethazine hydrochloride 4.7 45 94.3 20.1 9.6 Antihistamine Budesonide 9.9 > 66.7 > 66.7 > 20.1 > 6.7 Corticosteroid Tolterodine tartrate 10.3 88.8 > 200 > 19.4 8.6 Antimuscarinic Propafenone hydrochloride 5.3 53.8 102.2 19.3 10.2 Antiarrhythmic Defluoroxy paroxetine 5.2 40.7 101.2 19.3 7.8 Antidepressant Azelastine hydrochloride 6.2 33.3 99.5 16.1 5.4 Histamine antagonist Trihexyphenidyl hydrochloride 12.7 58.4 > 66.7 > 15.8 4.6 Antimuscarinic antiparkinsonian Propiverine hydrochloride 6.4 34.5 94.5 14.7 5.4 Muscarinic antagonist Phencynonate hydrochloride 10 48.9 146.3 14.6 4.9 Antimuscarinic Amitriptyline hydrochloride 6.5 43.7 93.2 14.3 6.7 Antidepressant Flutamide 14.6 > 200 > 200 > 13.7 > 13.7 Nonsteroidal antiandrogen Benproperine phosphate 6 30.4 82.4 13.7 5.1 Antitussive Cloperastine hydrochloride 6.5 35.8 84.5 12.9 5.5 Antitussive antihistamine Dicyclomine hydrochloride 9.3 48.7 114.9 12.3 5.2 Antimuscarinic Haloperidol 14.5 82.3 163.9 11.3 5.7 Antipsychotic Oxybutynin chloride 10.9 34.1 109.9 10 3.1 Antimuscarinic N-methylparoxetine 10.4 43.3 103 9.9 4.2 Antidepressant Ketotifen fumarate 23.7 108.7 > 200 > 8.4 4.6 Antihistamine Terfenadine 1 8.8 8.7 8.3 8.4 Antihistamine Clemastine fumarate 7.1 34.6 58.6 8.3 4.9 Antihistamine anticholinergic Fluphenazine 9.7 58.5 > 66.7 > 6.8 6 Antipsychotic Fluoxertine hydrochloride 5.7 35.8 37.4 6.6 6.3 Antipsychotic Paroxetine hydrochloride 5.1 28.2 33 6.4 5.5 Antidepressant Astemizole 1.9 9.3 11.1 5.8 4.9 Antihistamine Flupenthixol hydrochloride 6.8 26.1 34 5 3.8 Antipsychotic Amlodipine besylate 7.8 28 35.1 4.5 3.6 Calcium channel blocker, antihypertensive Table 1. In vitro anti-MARV activity of 33 hit compounds.
To test whether the anti-MARV activity that we observed in vitro can be also detected in vivo, we evaluated the effects of the 33 hit compounds in our previously established pseudovirus-based bioluminescent imaging (BLI) mouse model (Zhang et al. 2017) (see supplementary Materials and Methods for details of BLI analysis). Fol-lowing BLI analysis, 27 of the 33 compounds showed decreased p/HIV/MVGP/Fluc infection. The inhibitory effect was showed by the percentage of reduced BLI signal compared to the control group. Nineteen compounds showed an inhibitory effect over 30%, and 10 of these showed an inhibitory effect greater than 50% after treat-ment optimization (Fig. 1). As expected based on work by another group (Madrid et al. 2013), chloroquine phosphate and amodiaquine hydrochloride, which both showed high SI values, exhibited obvious protective effects, with inhi-bitory rates of 73% and 75%, respectively, when admin-istered daily from day 2 before infection until day 6 post-infection (Fig. 1A, 1B). During the BLI assay, the dosages and administration routes of the compounds were selected on the basis of equivalent human doses, as described in Supplementary Table S1. In order to mimic a clinically relevant treatment schedule—in which treatment is usually given after infection—most of the compounds were administered 4 h after virus inoculation, with a second dose given 1 day post-infection. However, due to the different properties of the different drugs, such as solubility, diffu-sion coefficient and half-life, the drug dosage and admin-istration timelines were further optimized according to the properties of each drug. Although chloroquine phosphate and amodiaquine hydrochloride exhibited obvious protec-tive effects, they only showed inhibitory rates of about 30% during our primary experiment when the drugs were administered post-infection only (data not shown), indi-cating that these two drugs may work better prophylacti-cally rather than therapeutically. Similarly, for mianserin hydrochloride and diltiazem hydrochloride, the inhibition efficacy was also increased substantially when daily doses were administered beginning 2 days prior to virus inocu-lation. As for the other drugs, further optimization of dosage and administration regimens may lead to better treatment efficacy, and this remains the subject of future investigation.
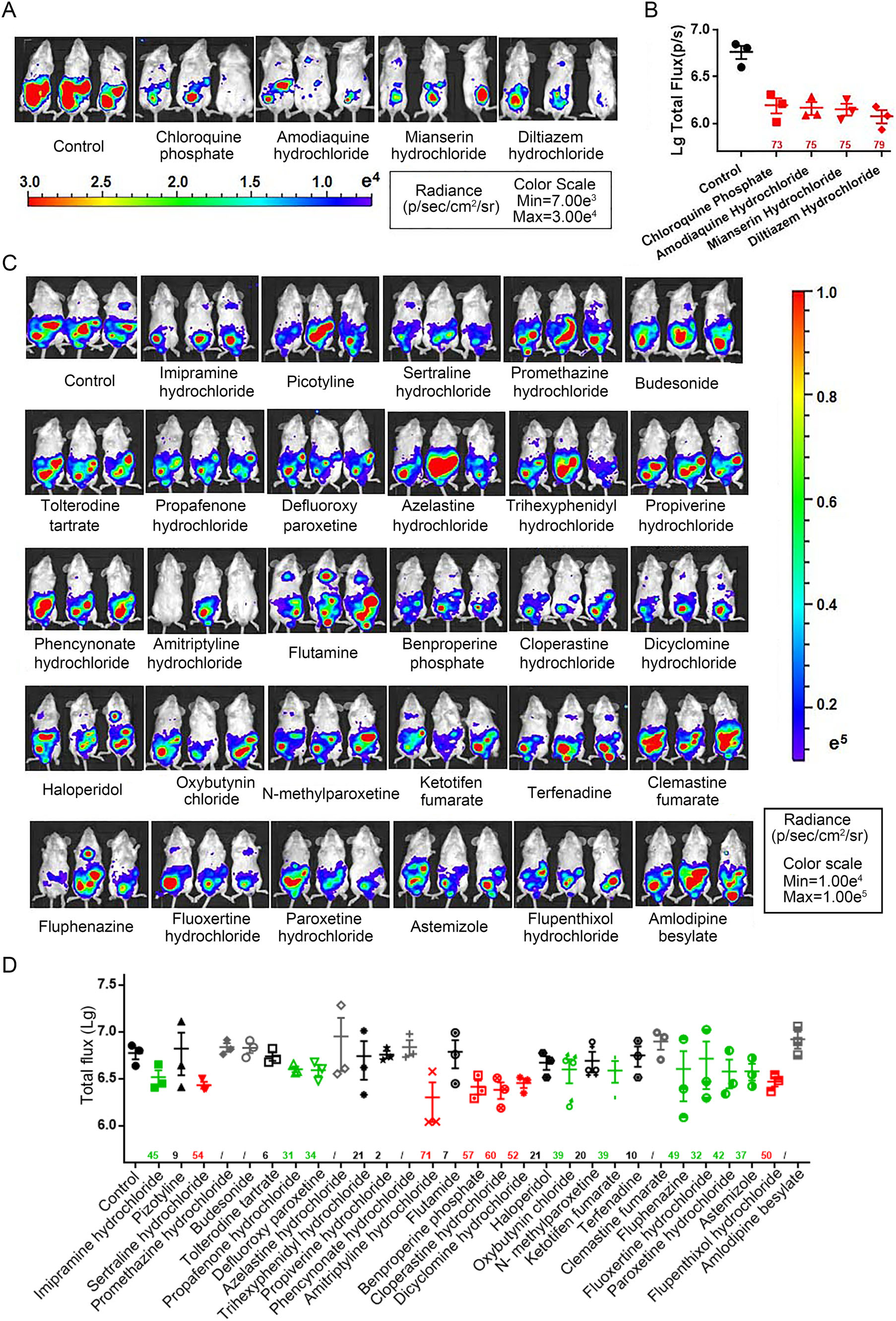
Figure 1. In vivo anti-MARV bioluminescent imaging (BLI) analyses. Four-to-five-week-old mice were injected with p/HIV/MVGP/Fluc (2.6×107 TCID50 in 200 μL PBS per mouse) via the IP route. The animals were treated with candidate anti-MARV compounds accord-ing to the procedure described in Supplementary Table S1. A, C Bioluminescence was measured 5 days post-infection and visual-ized in pseudocolor. B, D Total flux values for each compound. The percentage inhibitory rate is indicated on the X-axis and was calculated using the following equation: (1-geomean total flux of candidate drugs/geomean total flux of control) 9 100%. The per-centage inhibitor rates are color-coded as follows: Red > 70%, green > 50%, black > 0%, gray < 0%.
A critical consideration in the pre-clinical development of these drugs is whether they remain effective against authentic virus in a suitable animal model system. Due to the biosafety 4 lab limitation, we have not get these data yet. The candidate drugs may be assessed their efficacy against live MARV using the established mouse model of infection in the future study. Previous reports have shown that chloroquine, one of the candidates that we screened out, is effective against EBOV in a mouse model (Madrid et al. 2015), and against MARV in vitro (Madrid et al. 2013). Interestingly, chloroquine failed to protect guinea pigs from EBOV infection (Madrid et al. 2015). This may be related to decreased bioavailabilty and increased toxicity of the drug in this animal model. Future work should therefore focus on evaluating the efficacy of can-didate drugs in several animal models of MARV infection. Not only mouse model, but also guinea pigs and the hamster model will be expected to be tested (Marzi et al. 2016).
HTML
-
We are grateful to Zongge Zhao and Lihua Xie from the Institute for Reference Standards and Standardization, National Institutes for Food and Drug Control, for providing the compounds. This work was supported by the National Key Research and Development Plan of China (Grant No. 2016YFC1200904). The funding bodies had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
-
The authors report no conflicts of interest.
-
All institutional and national guidelines for the care and use of laboratory animals were followed.
















 DownLoad:
DownLoad: