HTML
-
Arboviruses are propagated via sensitive arthropods (e.g., mosquito, tick, sandfly, and midge), the bites of which transmit the viruses to humans and animals (Weaver and Reisen 2010). In 1992, the International Arbovirus Center registered 534 arboviruses, of which 128 can cause human and livestock diseases (Karabatsos 1985). More than 300 arboviruses are mosquito-borne (Karabatsos 1985), including the highly pathogenic dengue virus (DENV) (OMS 2000), Japanese encephalitis virus (JEV) (Erlanger et al. 2009), West Nile virus (Mackenzie et al. 2004), and Zika virus (ZIKV) (Franca et al. 2016). The 2015-2016 ZIKV epidemic in Brazil and other countries in South America spreaded to more than 60 countries, and approximately 2 million people were infected (Franca et al. 2016), some of whom developed congenital Zika virus syndrome (Mlakar et al. 2016), Guillain-Barre syndrome (GBS) (Cao-Lormeau et al. 2016), and non-vector-borne stillbirth (e.g., maternofetal, sexual, and post-transfusion) (Enserink 2015; Ogden et al. 2016; Rahman and Huhtaniemi 2017). Additionally, indigenous ZIKV infections occurred in Cambodia (Vireak et al. 2012), Thailand (Buathong et al. 2015), and Vietnam (Moi et al. 2017), occasionally resulting in microcephaly. The huge public health burden of ZIKV infection and the lack of vaccines and effective treatments have necessitated the gathering of information on the vectors and biological characteristics of ZIKV to promote infection prevention and control.
ZIKV was first isolated from the serum samples of rhesus monkeys from the Zika forest in Uganda in 1947; The prototype strain was MR766 (Karabatsos 1985). Since then, ZIKV has been isolated from mosquito specimens many times (Diallo et al. 2014; Epelboin et al. 2017; Guedes et al. 2017). In total, 31 strains of ZIKVs were isolated from mosquito specimens collected in Seychelles, 28 from 10 species of Aedes mosquitoes, and 1 each from Mansonia uniformis, Culex perfuscus, and Anopheles coustani (Diallo et al. 2014). From 1952 to August 12, 2017, ZIKV was isolated from 16 Aedes species (Epelboin et al. 2017), and Ae. aegypti is the leading vector of ZIKV (Epelboin et al. 2017). Thus, mosquito-borne arboviruses, especially zika viruses, are of serious public health concern.
Jiangxi Province encompasses the middle and lower reaches of the Yangtze River and contains many rivers and smaller waterways, as well as an extensive road network, which facilitates exchanges between Jiangxi Province and the surrounding provinces (People's Government of Jiangxi Province 2019). The rapid development of transportation and tourism has increased the risk of transporting viruses transmitted by blood-sucking insects from surrounding areas into the province. For example, outbreaks of DENV occurred in Guangdong Province (south of Jiangxi Province) (Zhang et al. 2014; Sun et al. 2016), and Akabane virus has been detected in mosquitoes in Hunan Province (Cao et al. 2019). To evaluate the situation of the arbovirus species and their distribution in Jiangxi Province, we collected blood-sucking insects in ten counties from June to July 2018. Six ZIKV strains were isolated from Culex tritaeniorhynchus and Anopheles sinensis. This is the first report of isolation of ZIKV from mosquito specimens in Jiangxi Province from Cx. tritaeniorhynchus and An. sinensis.
-
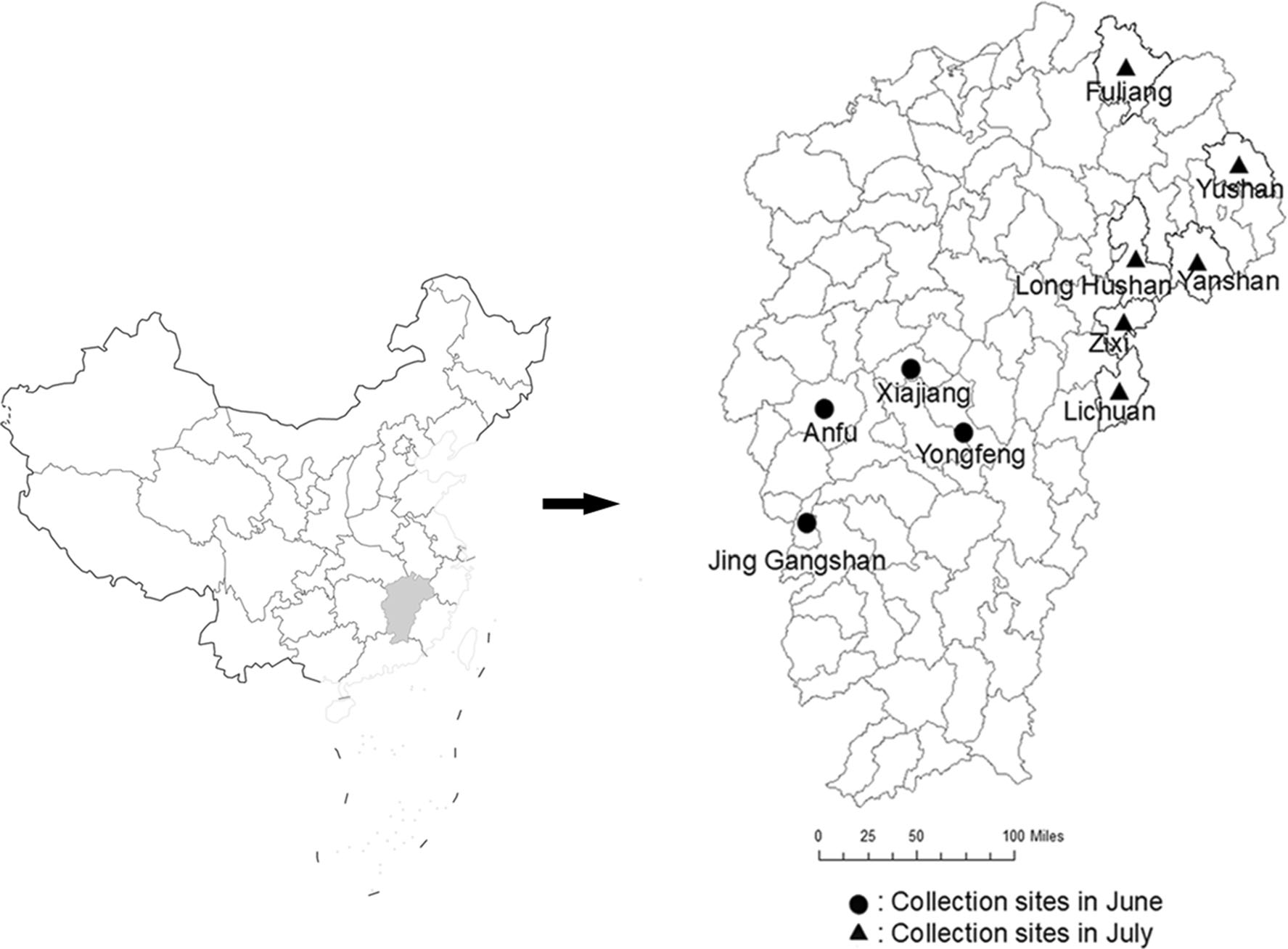
In June and July 2018, we collected blood-sucking insects (mosquitoes and midges), in Xiajiang, Yongfeng, Anfu, Jing Gangshan, Lichuan, Zixi, Yanshan, Yushan, and Fuliang counties and Long-Hushan Town of Jiangxi Province. The specimen collection sites were selected based on their suitability for the breeding of blood-sucking insects and human and livestock activities (e.g., housing and sheep, chicken, and pig farms). Insect specimens were collected using an ultraviolet mosquito-lured lamp (Wuhan Jixing Environmental Protection Technology Co., Ltd.) from 18:00 to 06:00. The mosquito specimens were classified according to their morphology under ice bath conditions, and numbered and registered according to the collecting environment and species. The specimens were stored in liquid nitrogen and transferred to the laboratory for testing (Song et al. 2017).
-
BHK-21 (golden hamster kidney cells) and C6/36 (Aedes albopictus oocytes) cells were cultured in 90% Eagle's medium (7% fetal bovine serum [FBS; Invitrogen], 1% penicillin-streptomycin [100 U/mL], 1% glutamine [30 g/L], and 1% NaHCO3) and 89% Roswell Park Memorial Institute 1640 medium (Invitrogen) (10% FBS [Invitrogen] and 1% penicillin-streptomycin [100 U/mL]) in a 5% CO2 incubator at 37 ℃ and 28 ℃, respectively (Fu et al. 2017; Song et al. 2017).
-
Mosquitoes were combined into pools of 50-100 and ground using a glass grinder. Each pool was washed twice with 1.5 mL of grinding fluid (93% Eagle's medium [laboratory preparation], 5% penicillin-streptomycin [100 U/mL], 1% glutamine [30 g/L], and 1% NaHCO3), then 1.5 mL of grinding fluid was added, and the sample was repeatedly ground in an ice bath. Next, the cells were centrifuged (20, 000 ×g, 4 ℃, 20 min), and 100 μL of the supernatant was collected and inoculated into 80% confluent BHK-21 and C6/36 cells in 24-well plates (Corning Inc.) and cultured in a 5% CO2 atmosphere at 37 ℃ and 28 ℃, respectively. The cytopathic effect (CPE) was evaluated under a microscope at 12-h intervals. Upon the appearance of CPE, the virus solution was collected and stored at -80 ℃. Specimens without CPE were blindly passaged in the above two cell lines for three generations, and those that did not show CPE were discarded (Fu et al. 2017; Song et al. 2017).
-
The pooled infection rate was calculated as follows: pooled infection rate = positive specimen pool number ÷ total number of pools of processed specimens.
Assuming that each positive pool contains only one infected mosquito, the minimum infection rate of 1000 mosquitoes = number of pools of positive specimens (number of infected mosquitoes) ÷ the total number of treated mosquitoes×1000 (Feng et al. 2012; Ren et al. 2017).
-
Total RNA was extracted from the specimens using a Viral RNA Mini Kit (Qiagen) according to the manufacturer's instructions. The extracted RNA sample was immediately placed in a 65 ℃ water bath for 10 min followed by an ice bath for 2 min. The RNA sample (32 μL) was transferred to a first-strand reaction tube (GE Healthcare, Little Chalfont, Buckinghamshire, UK), allowed to stand for 1 min at room temperature, and 1 μL of random primer (pd(N)6) was added. Finally, the sample was centrifuged, placed in a water bath at 37 ℃ for 60 min, and stored at -40 ℃.
-
Polymerase chain reaction (PCR) amplification was performed using cDNA as the template, 2×GoTaq® Green Master Mix (Promega, Madison, WI, USA), 10 μmol/L upstream and downstream primers for flavivirus genes (Wang et al. 2011), and the ZIKV coding region (ORF) gene (Song et al. 2017). Next, 5 μL of the PCR products was resolved by electrophoresis in 1% agarose gel and sequenced. The sequence of the coding region of ZIKV was obtained using an amplification primer of the whole ZIKV genome sequence, which was obtained from reference (Fu et al. 2017; Song et al. 2017).
The nucleotide sequences were aligned using the Basic Local Alignment Search Tool of the National Center for Biotechnology Information. Nucleotide sequence splicing and mass analysis were performed using Seqman software (DNAStar, Madison, WI, USA), and multiple sequence alignments were performed with BioEdit software (ver. 7.0, Thomas). Phylogenetic analysis by the neighbor-joining method with 1000 bootstrap replicates was performed using MEGA ver. 6.0 software. The homology of the nucleotide and amino acid sequences was analyzed using MegAlign (Fu et al. 2017; Song et al. 2017). The viral gene sequences are shown in the Supplementary file (Supplementary Table S1).
-
BHK-21 cells were transferred to a six-well culture plate (Corning Inc.) and cultured to 80% confluence. Virus suspensions (10-1-10-6 dilutions) were added to the sixwell culture plates (0.1 mL/well). After adsorption for 1 h at 37 ℃ in 5% CO2, 1.3% methylcellulose-MEM semisolid medium (5 mL/well) containing 2% FBS was added to each well. When plaques were visible under a microscope (3-5 days), the medium was discarded, the cells were stained with crystal violet, and the number of plaqueforming units (PFU) was calculated (Cao et al. 2016).
Specimen Collection
Cell Culture
Virus Isolation
Minimum Infection Rate
Viral RNA Extraction and cDNA Library Preparation
Nucleotide Sequence Determination and Viral Gene Analysis
Plaque Assay
-
In June and July 2018, in total, 22, 985 mosquitoes of four genera and four species (Cx. tritaeniorhynchus, An. sinensis, Cx. pipiens quinquefasciatus, and Armigeres subalbatus) and 57, 500 midges (types to be identified) were collected in ten counties/towns of Jiangxi Province (Fig. 1). There were 16, 592 Cx. tritaeniorhynchus and 5822 An. sinensis specimens (72.2% [16, 592/22, 985] and 25.3% [5822/22, 985] of the total mosquitoes, respectively) (Table 1). Cx. tritaeniorhynchus and An. sinensis are local dominant mosquitoes.
Period of sample collection Sampling site (county) Mosquito species Total Midges Culex tritaeniorhynchus Culex quinquefasciatus Armigeres subalbatus Anopheles sinensis June Xiajiang 3130
(1530/1600)a0 80 830
(730/100)3960
(2260/1700)3000 Yongfeng 5100
(2100b/3000)0 0 2600
(1250b/1350)7700
(3350/4350)1100 Anfu 530b 0 0 500b 1030 550 Jing 55 39 0 33 127 4550 Gangshan (1900/2650) Subtotal 8815
(4215/4600)39 80 3963
(2513/1450)12897
(6847/6050)9200
(6550/2650)July Lichuan 270 0 0 230 500 12400 Zixi 15 0 6 91 112 7800 Long-Hushan town 520 0 44 197b 761 8900 Yanshan 5150 0 370 1100 6620 10600 Yushan 1660 0 12 200 1872 5300 Fuliang 162 0 20 41 223 3300 Subtotal 7777 0 452 1859 10088 48300 Total 16592
(11992/4600)39 532 5822
(4372/1450)22985
(16935/6050)57500
(54850/2650)a(1530/1600): (number of ground mosquito specimens/number of unground mosquito specimens).
bZIKV isolated from this mosquito specimen.Table 1. Specimens of blood-sucking insects collected in Jiangxi Province in 2018.
-
Eleven virus isolates were stably passaged in tissue culture; these included several JEV and DENV strains (results not shown). Six ZIKV isolates, (JXJA1835-1, JXJA1835-2, JXJA1839-1, JXJA1866, JXJA1874-1, and JXLHS 1807) (Table 2), showed CPE and were stably passaged in tissue culture. Of these, five were isolated from specimens collected in June, and one from a specimen collected in July. Two of the six ZIKV strains were isolated from Cx. tritaeniorhynchus and four from An. sinensis (Table 2). The ZIKV isolate, JXLHS1807, from An. sinensis showed CPE in BHK-21 cells and formed plaques (Fig. 2).
Sampling date Sampling site (county) Strain Mosquito species Breeding ground GenBank Accession No. June 2018.06.12 Yongfeng JXJA1835-1 Anopheles sinensis Cowshed MK696546 2018.06.12 Yongfeng JXJA1835-2 Anopheles sinensis Cowshed MK696547 2018.06.12 Yongfeng JXJA1839-1 Culex tritaeniorhynchus Cowshed MK696548 2018.06.13 Anfu JXJA1866 Anopheles sinensis Sheepfold MK696549 2018.06.13 Anfu JXJA1874-1 Culex tritaeniorhynchus Cowshed MK696550 July 2018.07.10 Long-Hushan town JXLHS1807 Anopheles sinensis Pigsty MK696551 Table 2. ZIKV strains isolated from mosquitoes in Jiangxi Province in 2018.
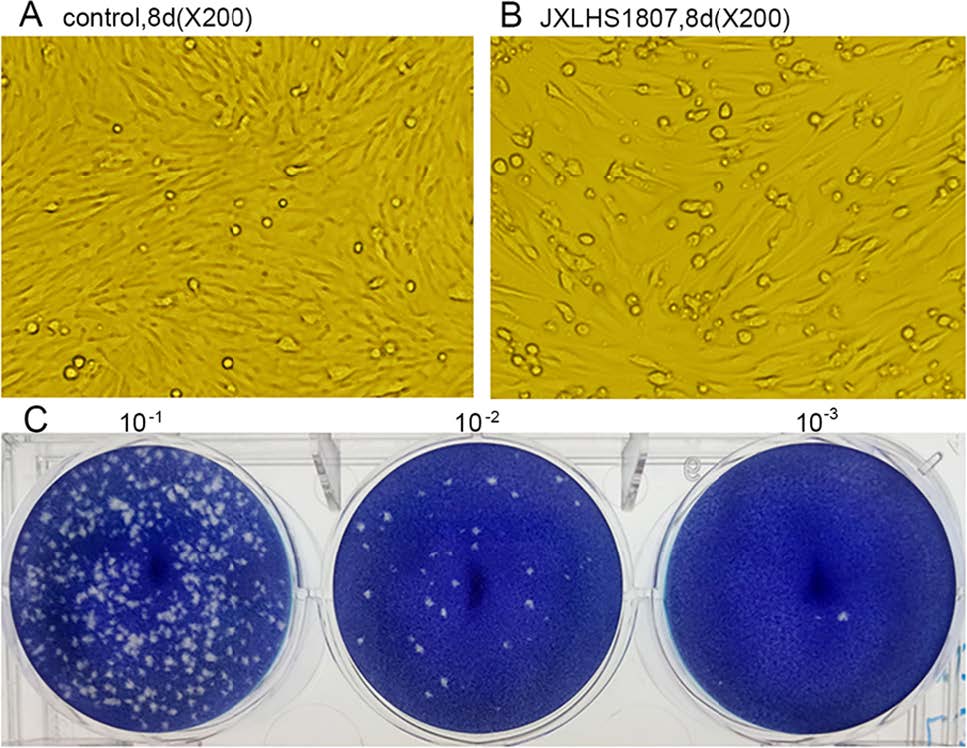
Figure 2. CPE and plaques caused by ZIKV (JXLHS 1807). Normal BHK-21 cells cultured for 8 days grew densely and were neatly arranged (A), whereas inoculation with ZIKV strain JXLHS1807 reduced the number of adherent cells and induced rounding and exfoliation (B). Manification, 200×. C Plaques in monolayers of BHK-21 cells caused by JXLHS1807 with different dilutions.
Four mosquito species (Cx. tritaeniorhynchus, An. sinensis, Cx. pipiens quinquefasciatus, and Ar. subbalbatus) were collected in Jiangxi Province. Among these, six ZIKV strains were isolated from Cx. tritaeniorhynchus and An. sinensis collected in Yongfeng County, Anfu County, and Long-Hushan Town. The minimum ZIKV infection rate of Cx. tritaeniorhynchus and An. sinensis was 0.76 and 2.05, respectively (Table 3).
Collection date Collection site (county) Mosquito species No. Individuals/no.pools No. Positive Pools/no.pools MIR(/1000) June Yongfeng Culex tritaeniorhynchus 2100/14 1/14 0.48 Yongfeng Anopheles sinensis 1250/8 2/8 1.60 Anfu Culex tritaeniorhynchus 530/5 1/5 1.89 Anfu Anopheles sinensis 500/5 1/5 2.00 July Long-Hushan town Anopheles sinensis 197/4 1/4 5.08 Subtotal Culex tritaeniorhynchus 2630/19 2/19 0.76 Anopheles sinensis 1947/17 4/17 2.05 Table 3. ZIKV infection rate of Cx. tritaeniorhynchus and An. sinensis.
-
The number of nucleotide and amino acid sequences of the E gene (Li et al. 2006; Lin et al. 2008) of the six ZIKV strains (JXJA1835-1, JXJA1835-2, JXJA1839-1, JXJA1866, JXJA1874-1, and JXLHS 1807) were 1510 nt and 504 aa, and similarity among the six strains was 99.7%-100.0% and 99.2%-100.0%, respectively.
The nucleotide and amino acid sequences (Li et al. 2006; Lin et al. 2008) of the JXLHS1807 ORF were 10, 296 nt and 3423 aa in length, respectively, and encoded a single reading frame. The details of the six ZIKV strains are listed in Table 2.
The nucleotide and amino acid sequence homologies of the ORF of JXLHS1807 and other ZIKV isolates were 88.4% (African type 1 MR766, Uganda), 88.4% (African type 2 ArD7117, Senegal), 96.2% (Africa type 1 MR766, Uganda), and 96.3% (African type 2 ArD7117, Senegal). The corresponding levels of homology with Asian ZIKV strains were 95.3% (Asian type 1 P6-740, Malaysia), 99.7% (Asian type 2 SZ-WIV01, American Samoa), 98.4% (Asian type 1, P6-740, Malaysia), and 99.5% (Asian type 2 SZ-WIV01, American Samoa) (Table 4).
Genotype Strain Source Homology of nucleotide (amino acid) of virus Divergences of amino acids Country Year Host JXLHS1807(ORF) JXLHS 1807(E) D683E V763M T777M S139N M/t2634V Africa Ⅰ MR766 Uganda 1947 Rhesus money 88.4% (96.2%) D V T s M Africa Ⅱ ArD7117 Senegal 1968 Aedes luteocephalus 88.4% (96.3%) D V T s M Asia Ⅰ P6-740 Malaysia 1966 Aedes aegypti 95.3% (98.4%) D V T s T Asia Ⅰ GZDJ1666-2 China 2016 Armigeres subalbatus 99.9% (99.7%) E M M N M Asia Ⅱ GZDJ1685 China 2016 Culex quinquefasciatus 99.9% (99.7%) E M M N M Asia Ⅱ SZ-WIV01 Americ an-S amon 2016 Human 99.7% (99.5%) E M M N M Asia Ⅱ ECMN2007 Micronesia 2007 Human 97.8% (98.8%) E V T S M Asia Ⅱ H/PF/2013 French-Polynesia 2013 Human 99.5% (99.4%) E M M N M Asia Ⅱ 1_0035_PF French-Polynesia 2014 Human 99.4% (99.4%) E M M N M Asia Ⅱ ZikaSPH2015 Brazil 2015 Human 99.3% (99.2%) E M M N V Asia Ⅱ JXLHS1807a China 2018 Anopheles sinensis E M M N M Asia Ⅱ JXJA1835-1a China 2018 Anopheles sinensis 99.7% (99.2%) E M M Asia Ⅱ JXJA1835-2a China 2018 Anopheles sinensis 99.8% (99.4%) E M M Asia Ⅱ JXJA1839-1a China 2018 Culex tritaeniorhynchus 99.8% (99.4%) E M M Asia Ⅱ JXJA1866a China 2018 Anopheles sinensis 99.8% (99.4%) E M M Asia Ⅱ JXJA1874-1a China 2018 Culex tritaeniorhynchus 99.8% (99.4%) E M M aZIKV strains isolated in this study. Table 4. Variation in the amino acid sequences of ZIKVs isolated from Cx. tritaeniorhynchus and An. sinensis.
-
The amino acid substitutions S139N in the PrM protein; D683E, V763M, and T777M in the E protein; and M/t2634V in the NS5 protein of JXLHS1807 were identical to those of the ZIKV strains prevalent in South America since 2015. The E proteins of the other five ZIKV strains isolated from Cx. tritaeniorhynchus and An. sinensis (JXJA1835-1, JXJA1835-2, JXJA1839-1, JXJA1866, and JXJA1874-1) had the D683E, V763M, and T777M amino acid substitutions (Table 4).
-
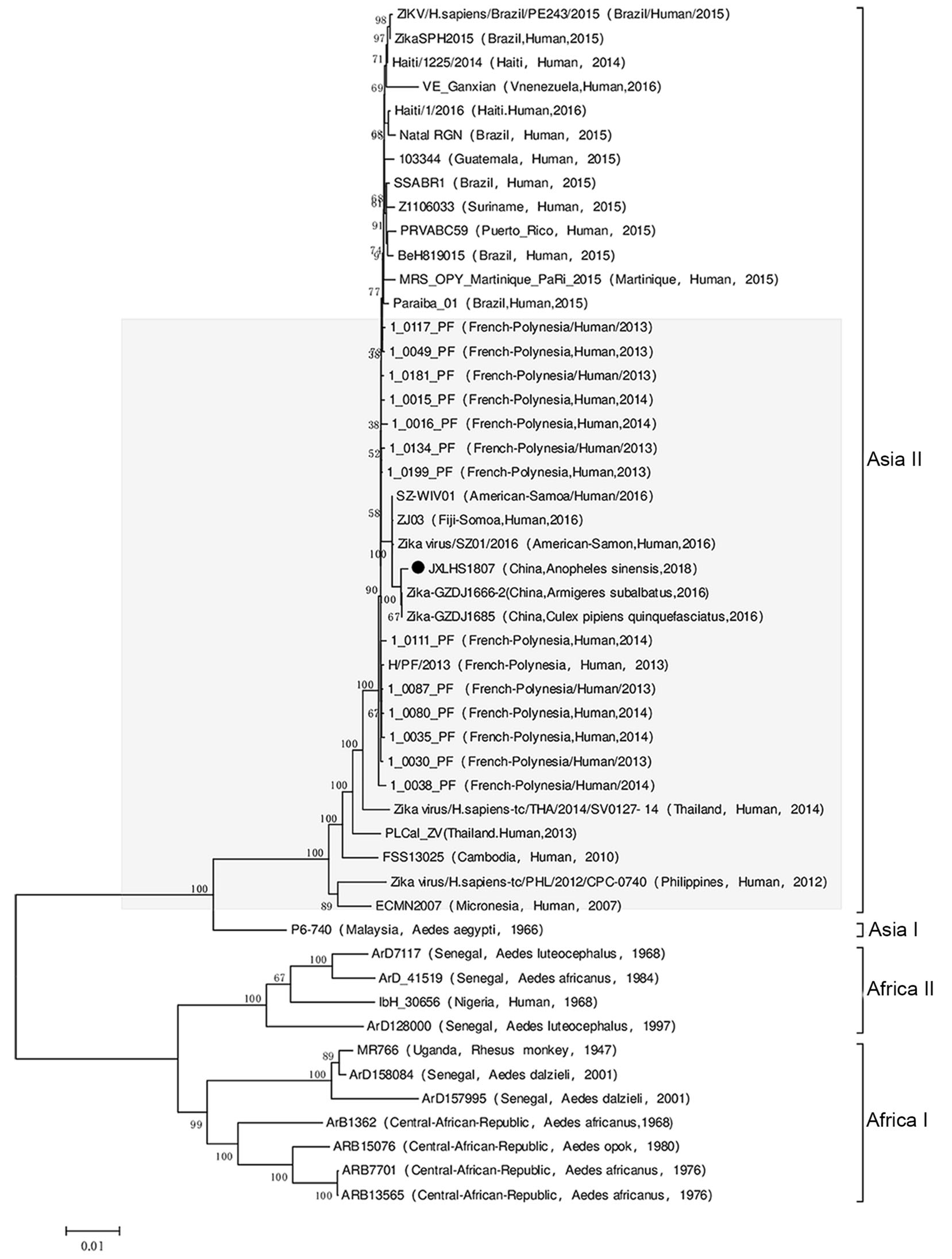
A molecular phylogenetic analysis based on the viral open reading frame nucleotide sequences showed that JXLHS1807 was an Asian type 2 ZIKV, as those isolated from Cx. pipiens quinquefasciatus (GZDJ 1685) and Ar. subbalbatus (GZDJ1666-2) collected in Guizhou Province in 2016. And Asian type 2 ZIKVs were also isolated in Yap Island (ECMN2007, Micronesia), French Polynesia (1_0199_PF, French Polynesia), Brazil (SSABR1, Brazil), Puerto Rico (PRVABC59, Puerto Rico), and Haiti (Haiti 1225/2014, Haiti) since 2007. However, JXLHS1807 was not in the same evolutionary branch as the ZIKV strains prevalent in South America since 2015 (Fig. 3).
Insect Specimens
Virus Isolation and Mosquito Infection of ZIKV
Molecular Biological Characteristics of the ZIKV Strains
Nucleotide Sequence Analysis
ZIKV Amino Acid Sequence Variation
Molecular Phylogenetic Analysis of the ZIKV Strains
-
A variety of Aedes species, particularly Ae. aegypti and Ae. Albopictus, can be infected by ZIKV, which can be detected in their salivary glands, suggesting that they are vectors for ZIKV (Lourenco-de-Oliveira and Failloux 2017). Indeed, ZIKV can be detected in the salivary glands of Cx. pipiens quinquefasciatus after artificial infection (Guo et al. 2016; Guedes et al. 2017). ZIKV strains have also been isolated from An. coustani, An. gambiae, Cx. perfuscus, and M. uniformis (Diallo et al. 2014; Epelboin et al. 2017), Cx. pipiens quinquefasciatus (Guedes et al. 2017; Song et al. 2017), and Ar. subbalbatus (Fu et al. 2017), as well as from Cx. tritaeniorhynchus and An. sinensis in this study (Table 2). Therefore, Cx. perfuscus, An. coustani, Ar. subbalbatus, M. uniformis, Cx. pipiens quinquefasciatus, Ar. subbalbatus, An. sinensis, and Cx. tritaeniorhynchus also carry ZIKV and are potential secondary vectors (Epelboin et al. 2017).
An. sinensis is widely distributed throughout mainland China, except Xinjiang and Qinghai (18°10'-53°33' N, 103°04'-135°2'30" E). Female An. sinensis feed on the blood of humans and livestock, with a preference for the blood of large livestock species such as cattle, horses, and donkeys. Rice fields are the main breeding grounds for An. sinensis, but they also proliferate in swamps, reed fields, lakesides, channels, ponds, and depression ponds. An. sinensis is the main vector of malaria in China (Ma 1981; Ren et al. 2015). An. sinensis in China also carries the Getah virus (GETV), Banna virus (BAV, Seadornavirus), Liaoning virus (LNV), Kadipiro virus (KDV), and DNV (Liang et al. 2018). The geographical distribution of Cx. tritaeniorhynchus in China is similar to that of An. sinensis, and is also found in eastern, southern, and southeastern Asia. Female Cx. tritaeniorhynchus mosquitoes feed on the blood of humans and animals (typically pigs and cattle) (Miller et al. 2012; Gould et al. 2017). Cx. tritaeniorhynchus is the major vector of JEV (Miller et al. 2012; Liang et al. 2018). In addition, in China, Cx. tritaeniorhynchus carries an alphavirus (GETV), flaviviruses (JEV, Tambosu virus, and mosquito-borne flavivirus), bunyaviruses (AKV and Cat Que virus), BAV (genus Seadornavirus, family Reovirus), LNV, KDV, orbivirus (Yunnan orbivirus), parvovirus (DNV), and ten other viruses (Liang et al. 2018). An. sinensis and Cx. tritaeniorhynchus are the dominant mosquito species in mainland China and carry the largest number of arboviruses (Liang et al. 2018). We report here the first isolation of ZIKV from Cx. tritaeniorhynchus and An. sinensis.
The ZIKV strains isolated from Cx. tritaeniorhynchus and An. sinensis in Jiangxi Province in 2018 were in the same evolutionary branch as those isolated from Cx. pipiens quinquefasciatus and Ar. subbalbatus in Guizhou Province in 2016, suggesting that despite the geographic (> 1000 km) and temporal (2 years) separation of the ZIKV strains isolated in Jiangxi and Guizhou Provinces, they originate from the same evolutionary population. Indeed, the ZIKV isolated in Guizhou Province in southwestern China in 2016 and the ZIKV isolated in Jiangxi Province in southeastern China in 2018 may have been transmitted to China simultaneously. The phylogenetic analysis suggests that the ZIKV strains isolated in Guizhou Province in 2016 and Jiangxi Province in 2018 are in the same evolutionary cluster as those isolated in Micronesia in 2007 (ECMN 2007) and French Polynesia in 2014 (1- 0016_PF) (Fig. 3). Therefore, the ZIKV strains isolated from Cx. pipiens quinquefasciatus, Ar. subbalbatus, An. sinensis, and Cx. tritaeniorhynchus in China from 2016-2018 entered the country during or before 2016.
One ZIKV strain each was isolated from Cx. pipiens quinquefasciatus and Ar. subbalbatus specimens collected in Guizhou Province, China, in 2016 (Fu et al. 2017; Song et al. 2017). In the PCR detection of the ZIKV gene in all the collected mosquitoes, only the pools of mosquito specimens isolated from the virus were positive for ZIKV PCR (Fu et al. 2017; Song et al. 2017). In this study, six ZIKV strains were isolated from An. sinensis (four strains) and Cx. tritaeniorhynchus (two strains) specimens collected in Jiangxi Province, China. The infection rate of ZIKV in this survey in Jiangxi Province was 0.76 per 1000 Cx. tritaeniorhynchus, and the infection rate of ZIKV per 1000 An. sinensis was 2.05. In particular, the infection rate of ZIKV (JXLHS 1807) isolated from An. sinensis collected in July was 5.08 per 1000 An. sinensis, which indicates that the infection rate of ZIKV per 1000 An. sinensis may be higher than 2.05 and significantly higher than the ZIKV infection rate of An. sinensis in other areas during the same period in 2018 (Table 3). The high infection rate of ZIKV in An. sinensis and Cx. tritaeniorhynchus raises a new research topic regarding whether ZIKV can infect local animals or humans. ZIKV is an arbovirus circulating between Aedes and non-human primates (Epelboin et al. 2017; Lourenco-de-Oliveira and Failloux 2017), which raises the following questions: (1) non-human primates are known to be ZIKV hosts in nature, but recent results suggest that pigs and rabbits may be potential hosts for ZIKV (Ragan et al. 2017). Since almost all households in rural areas of Jiangxi province raise pigs, and most of the specimen collection points in this study are pigsties and cow enclosures, it is necessary to conduct a study on ZIKV infection in local domestic animals and investigate whether there is natural circulation between mosquitoes and animals such as pigs or cows. (2) Are there any cases of ZIKV infection in humans in the local area? There have been cases of imported ZIKV infection in China, but no local infections have been reported (Li et al. 2016). Since most ZIKV infections present only mild manifestations such as fever or fever with rash, these manifestations may also be overlooked or misdiagnosed as DENV or JEV infection. A recent study reported that the frequency of ZIKV co-infection with other classical flaviviruses was higher than that of its single infection (Duong et al. 2017). Therefore, it is necessary to strengthen the detection of ZIKV infection in arbovirus-infected patients in Jiangxi province in the summer, such as the detection of ZIKV and DENV co-infections, to determine whether there are ZIKV-infected patients in the local area. Finally, given that ZIKV has been isolated from Cx. pipiens quinquefasciatus (Song et al. 2017), Ar. subbalbatus (Fu et al. 2017), An. sinensis, and Cx. tritaeniorhynchus collected in the wild in China, it is necessary to conduct an artificial infection study on these mosquitoes to clarify whether they can be ZIKV vectors and to assess their public health significance in ZIKV transmission.
-
This work was supported by grants from the National Science and Technology Major Project (2018ZX10711001, 2018ZX1010 2001); The National Key Research and Development Program of China (2017YFC1200202); Science and Technology Project of Jiangxi (2014BBG70097) and Development Grant of State Key Laboratory of Infectious Disease Prevention and Control (2014SKLID103, 2015SKLID505). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the article.
-
JW, HX, SS and RC collected specimens, performed the experiments, analyzed the data and drafted the paper; NF, SF helped to perform the experiments and analyze the data; SZ, ZX, YH, WL and FL helped to collect specimens and analyze the data; HW, XL, GL conceived and designed the research study, analyzed the data and finalized the paper. All authors read and approved the final version of the paper.
-
The authors declare that they have no conflict of interest.
-
All animal experiments were conducted in strict compliance with the regulations set by the Animal Ethics Committee of China CDC.
















 DownLoad:
DownLoad: