-
Dear Editor,
The world is facing an immense challenge in the fight against coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Gorbalenya et al. 2020). To date, tens of millions of people worldwide have been infected with SARS-CoV-2, and more than a million have died, and the situation continues. (Huang et al. 2021; Li et al. 2021) A retrospective study in the nick of time is necessary to renew knowledge against the disease. Cytokine release syndrome (CRS) features a dramatic increase of various pro-inflammatory cytokines and has been highlighted in COVID-19 patients; CRS is thought to be responsible for severe illness and high mortality in this disease (Moore and June 2020; Ruan et al. 2020). Therefore, cytokine concentrations may be useful for predicting the clinical outcomes of COVID-19, and anti-inflammatory strategies intended to regulate the cytokine pathway in COVID-19 patients are considered to be an alternative therapeutic route (Fu et al. 2020; Guan et al. 2020; Mehta et al. 2020).
Among CRS-related cytokines, interleukin-6 (IL-6), as a key mediator, has been a particular focus in COVID-19 infection (Aziz et al. 2020). IL-6 is a known pleiotropic cytokine with complex biological functions (Velazquez-Salinas et al. 2019). It plays an essential role in activating and/or regulating various cell populations upon infections. However, the overexpression of IL-6 might promote disease progression. Several studies have suggested that the level of IL-6 is closely associated with COVID-19 progression because elevated IL-6 can be observed in severe and critically ill patients (Aziz et al. 2020; Chen et al. 2020b). In summary, elevated IL-6 has been identified as a predictor of COVID-19 severity (Han et al. 2020; Liu et al. 2020a; Ulhaq and Soraya 2020; Zhu et al. 2020), mechanical ventilation (Herold et al. 2020), and mortality risk (Laguna-Goya et al. 2020; Ruan et al. 2020; Zhou et al. 2020). Over the past few months, significant efforts have been made to clarify the relevance and roles of IL-6 in COVID-19. However, a robust analysis based on a large sample size has not yet been conducted. Moreover, most previous studies have been conducted based on single measurements of IL-6, and few studies have explored dynamic changes of IL-6 concentrations in COVID-19 patients (Han et al. 2020; Liu et al. 2020b).
In this retrospective study, a total of 1,453 confirmed COVID-19 patients who were admitted to Huoshenshan Hospital from 29 February to 14 March 2020 were enrolled, including 962 (66%) moderate cases, 462 (32%) severe cases, and 29 (2%) critical cases. The demographics and clinical characterizations of the patients were listed in Supplementary Table S1. In this COVID-19 patient cohort, age was significantly associated with disease severity. There was no gender disparity; however, compared with women, more men had severe (53%) or critical (72%) illness. A relatively high percentage of patients had hypertension (23%; P < 0.001) or diabetes (12%; P < 0.001). In this cohort, hypertension and diabetes were significant risk factors for COVID-19.
Serum IL-6 concentrations in all patients were quantitatively determined using the Elecsys IL-6 detection kit (Roche Ltd). Concentrations of IL-6 in assorted groups were statistically analyzed and presented as the median (interquartile range; IQR). The median IL-6 concentrations for the moderate, severe and critical groups were 1.50 pg/mL (1.50–3.65 pg/mL), 2.60 pg/mL (1.50–9.03 pg/mL) and 60.92 pg/mL (10.49–408.80 pg/mL), respectively. Remarkably, the critical patients had significantly higher IL-6 concentrations compared with the moderate and severe patients (P < 0.001). Overall, 129 (13.4%) of the moderate patients, 125 (27.1%) of the severe patients, and 25 (86.2%) of the critical patients had IL-6 values that were greater than the reference concentration (7 pg/mL). These results indicate that elevated IL-6 is associated with COVID-19 severity (Han et al. 2020) and this biomarker might be a predictor of critical illness.
Furthermore, consecutive IL-6 concentrations were recorded for 184 patients (729 total tests) over approximately one month during hospitalization. These patients included 62 moderate patients, 111 severe patients, and 11 critical patients. Among them, 161 patients were cured, 11 patients were deceased, and 12 patients were discharged. None of these 184 patients received tocilizumab therapy. The dynamic changes in IL-6 corresponded to the COVID-19 severity and clinical outcomes of the patients, as shown in Fig. 1. In the moderate group, 58 patients (93.5%) were cured, 3 patients (4.8%) were discharged and one patient (1.6%) died. The total median IL-6 concentration of the moderate group was within the reference range throughout the monitoring period, while the one deceased patient demonstrated a remarkable increase in IL-6 from 43.44 to 5000 pg/mL (Fig. 1A). In the severe group, 100 patients (90.1%) were cured, 9 patients (8.1%) were discharged and two patients (1.8%) died. Similar to the moderate group, the median IL-6 concentrations slightly fluctuated near the reference concentration over time. The two deceased patients had high IL-6 concentrations that were above the reference value. It should be noted that the one patient who showed significant fluctuation in IL-6 that increased first and then decreased was cured at last (Fig. 1B). In the critically ill groups, 8 patients (72.7%) who had significantly increased IL-6 concentrations died, and 3 patients (27.3%) who showed no significant changes in IL-6 finally were cured (Fig. 1C). Based on the above results, we believe that the IL-6 concentration is closely related to disease progression, and in particular, dynamic monitoring of changes in IL-6 serum concentration may be helpful in predicting the development of COVID-19 regardless of clinical classification.
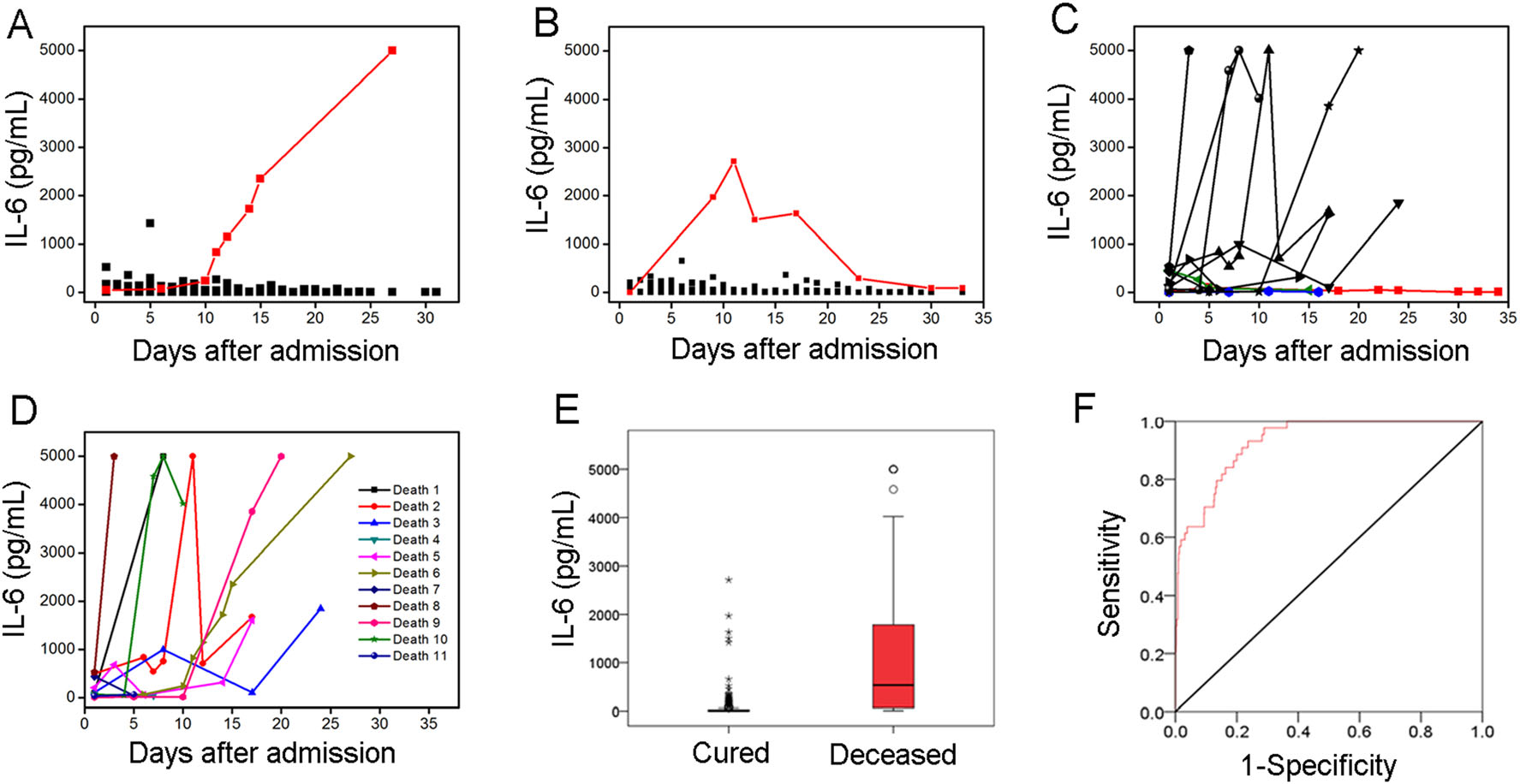
Figure 1. Evaluation of IL-6 changes in 184 patients with confirmed COVID-19. A Dynamic changes in IL-6 concentration of 62 moderate patients after admission. One deceased patient with a remarkable increase in IL-6 concentrations was highlighted in red. B Dynamic changes in IL-6 concentration of 111 severe patients after admission. One cured case with IL-6 increased first and then decreased was highlighted in red. C Dynamic changes in IL-6 concentration of 11 critically ill patients after admission. Three cured patients with slight change in IL-6 were highlighted in blue, green and red respectively. D Dynamic changes in IL-6 concentration of 11 deceased patients. E IL-6 levels in cured and deceased patient. F ROC curve of IL-6 for predicting COVID-19 mortality.
The dynamic changes in IL-6 concentrations corresponding to the clinical outcomes were further evaluated. All 11 deceased patients had high IL-6 concentrations, reaching up to 5,000 pg/mL in 54.5% of these cases (Fig. 1D). This finding indicates that a sharply elevated IL-6 concentration may be an important warning index of mortality (Chen et al. 2020a; Grifoni et al. 2020; Ruan et al. 2020; Zhou et al. 2020). The IL-6 concentrations of the 161 cured patients (655 total tests) and the 11 deceased patients (44 total tests) were statistically analyzed (Fig. 1E). The median IL-6 concentrations of the cured and deceased groups were 3.43 pg/mL (1.50–26.44 pg/mL) and 534.10 pg/mL (64.20–1, 815.50 pg/mL), respectively. The deceased group clearly showed a higher mean IL-6 concentration than the cured group. In addition, receiver operating characteristic curve (ROC) was used to evaluate the ability of IL-6 concentration to predict COVID-19 outcomes (Fig. 1F). The area under the curve was 0.93 (95% CI = 0.90–0.96), and the optimal IL-6 cutoff value was 31.57 pg/mL, with a sensitivity of 93.2% and a specificity of 76.3% according to the maximum Youden index. In our cohort, IL-6 concentrations greater than 31.57 pg/mL were associated with elevated mortality risk, with a positive predictive value of 0.21 and a negative predictive value of 0.99. The above results further suggest the availability of IL-6 to predict the prognosis of COVID-19.
In conclusion, serum IL-6 concentrations in COVID-19 patients were evaluated in assorted groups. Overall, 13.4% of moderate patients, 27.1% of severe patients, and 86.2% of critical patients had elevated IL-6 concentrations, suggesting a correlation between IL-6 and disease severity. Notably, the dynamic changes in IL-6 concentration corresponded to COVID-19 severity and clinical outcomes. The majority of the moderate and severe patients showed no significant changes in IL-6 levels over time, and the final cure rates in these patient groups were 93.5% and 90.1%, respectively. In contrast, the critically ill patients showed obviously increased IL-6 concentrations, which was associated with a high fatality rate of 72.7%. Overall, the deceased patients demonstrated a fairly high IL-6 level compared with the cured patients. Although the relationship between elevated IL-6 and COVID-19 progression is not entirely clear, these findings suggest that changes in the IL-6 concentration could be a good predictor of COVID-19 prognosis. We suggest that the dynamic changes in IL-6 should be monitored throughout the disease duration. This monitoring may have predictive value for changes in disease severity, as well as clinical value for applying timely and appropriate treatment.
HTML
-
This work was partially supported by the National Key Research and Development Program of China (Grant No. 2020YFC0861100), the National Natural Science Foundation of China (Grant No. 82002744), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB29050100), the Scientific Research Fund of Traditional Chinese Medicine of Hubei Health Commission (Grant No. ZY2021M035), the Youth Innovation Promotion Association of CAS (Grant No. 2014308) and the Interdisciplinary Innovation Team of CAS.
-
The authors declare that they have no conflict of interest.
-
Additional informed consent was obtained from all patients for which identifying information is included in this article.
















 DownLoad:
DownLoad: