-
The ideal strategy for viral prevention is an effective vaccine. Unfortunately, the development of a prophylactical vaccine against HIV-1 has been complicated by a number of factors such as the virus genomic variability, lack of a good animal model and lack of satisfactory surrogate markers of protective immunity, as well as the persistent nature of infection. Most importantly, HIV-1 has evolved astute mechani-sms to escape antibody and CTL responses (50, 60, 65). Despite intensive research since HIV-1 was disco-vered more than 25 years ago, there has been no success in developing a protective AIDS vaccine. In two large clinical trails finished in 2003 and 2007, respectively, neither VaxGen's gp 120 vaccine nor Merck's T cell-based vaccine showed protection. Cur-rently, multidrug combination therapies are the main strategy of HIV-1 management. Among these, protease and reverse transcriptase inhibitors, which target HIV-1 at postentry level, have had a major impact on HIV disease progression. However, the combined therapies are limited by their failure to eradicate virus, signifi-cant toxicities and the emergence of drug-resistant viral variants. Moreover, these drugs are not widely avai-lable in the developing world due to their high cost and the lack of adequate infrastructure for delivery. Thus, there is a need to identify and develop new classes of drugs to target additional stages of HIV-1 replication. Three major steps are involved in HIV-1 entry (Fig. 1): interaction of HIV-1 gp120 with cell surface CD4, gp120-CD4 complex binding with a coreceptor and membrane fusion mediated by the HIV-1 transmem-brane gp41 (3, 26, 27). Theoretically, compounds against these three steps can be considered as potential inhibitors of HIV-1 infection (Fig. 1 and Table 1) (24, 52, 68).
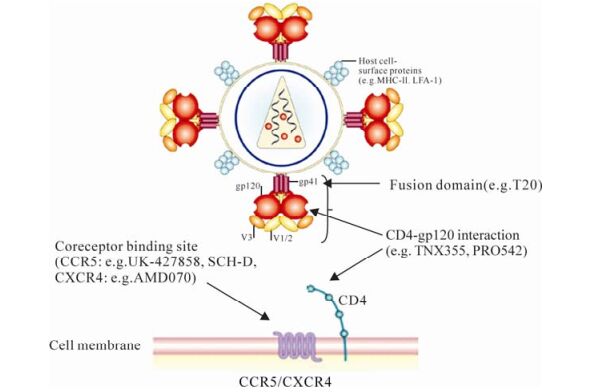
Figure 1. Potential targets for HIV-1 entry inhibition. T20 (enfuvirtide) is in clinical use. TNX-355, PRO-542, UK-427857 (maraviroc), SCH-D (vicriviroc) and AMD070 are currently in phase Ⅱ clinical trials.

Table 1. Entry inhibitors and the mechanisms of inhibitions
HTML
-
CD4 has been targeted for blocking HIV-1 entry since it was identified as the primary receptor of HIV-1 (12, 37). Antibodies to CD4 are among the most potent anti-HIV-1 compounds identified, but potential immunosuppressive effects limit their use for therapy (76). Nonetheless, a humanized anti-CD4 mAb, termed TNX355 (Tanox-Biogen, Houston, TX, USA), is now in clinical trials and seems not to cause immunosuppression (62). Other HIV inhibitors an-tagonizing CD4 pathway include small molecule NSC-13778 which binds to the N-terminal two-domain of CD4 (92) and CADA which down-regulates the CD4 receptor expression at post-translational level (86, 87).
-
Because CD4 has nanomolar affinity for gp120 and micromolar or lower affinity for MHC class Ⅱ, soluble mimics of CD4 represent attractive candidates for therapy. However, soluble CD4, which acts as a receptor decoy to prevent HIV-1 envelope glyco-protein (Env) engaging with CD4, showed no signi-ficant antiviral action in vivo despite its efficient neutralization in vitro (8, 11, 29). Nonetheless, PRO-542 (CD4-IgG2; Progenics Pharmaceuticals, Tarrytown, NY, USA), a tetrameric fusion protein between CD4 and immunoglobulin-G, is much more potent in vitro inhibitory activity than the parental monomer (31). When administered intravenously to HIV-1-infected adults and children at single and multiple doses ranging to 10 mg/kg, PRO-542 demon-strated excellent tolerability and pharmacology, and mediated statistically significant acute reductions in viral load (31, 69). In addition to PRO-542, other low-molecular-weight CD4 mimics include NBD-556 and NBD-557 (67, 94). A small molecule, named BMS-378806 (Bristol-Myers Squibb, Wallingford, CT, USA), has selective inhibitory activity for HIV-1 but not SIV and HIV-2 (44). Of note, BMS-378806 blocks the gp120-CD4 binding event, representing the first small molecule to inhibit this interaction (49). BMS-488043, an analogue of BMS-378806, demonstrated anti-HIV efficacy when it was orally administered (22, 33).
-
DC-SIGN (CD209), which is expressed on den-dritic cells (DCs), binds to gp120 with high affinity in addition to its natural ligand ICAM-3 (20). The interaction of HIV-1 with DC-SIGN does not result in infection of DCs, but instead enhances infection in trans of target cells that express CD4 and chemokine receptors. Since DCs normally traffic between mucosal surfaces and lymph nodes, it has been proposed that HIV-1 uses DCs as carriers, allowing the virus to access lymphoid tissue. DC-SIGN may play a critical role in mucosal transmission of virus. If so, inhibitors of DC-SIGN (e.g., monoclonal anti-bodies, sDC-SIGN) could serve as topical microbi-cides to bock sexual transmission of HIV-1 (24). In addition, HIV-1 gp120 glycoprotein can bind to heparin sulphate proteoglycans (HSPGs), glycolipid galactocerebroside (GalCer) and several other C-type lectin receptors (e.g., mannose receptor, langerin). HIV-1 can also attach to different cell types using the ancillary, cell-derived proteins that are incorporated into its membrane as the virus buds out of infected cells. Although it is still uncertain whether, and to what extent, these attachment receptors are involved in the transmission of HIV-1 in vivo, these cellular molecules could be potential targets for interrupting HIV-1 transmission (68).
Targeting CD4 receptor
Against CD4 binding sites on gp120
Other attachment targets
-
The discovery of chemokine receptors as corecep-tors for HIV-1 has led to the search for compounds to block HIV-1 infection. Due to the importance of CCR5 in transmission and during the asymptomatic phase of HIV-1 infection (10, 23), as well as the limited impact of a loss of CCR5 function, CCR5 inhibitors are likely to be successful anti-HIV drugs. The natural chemokine ligands are candidates for use in therapy and have been shown to repress HIV-1 replication in vitro (4, 9, 58). However, concerns about chemokines as inflammatory cytokines will prevent their clinical use. Accordingly, modified chemokine antagonists and small molecules that bind to the desired receptor without triggering intracellular signalling have been the major goal in the search for coreceptor-targeted inhibitors. The N-terminal modified RANTES, aminooxypentane-RANTES (AOP-RAN TES), was identified and showed potent inhibition of R5 HIV-1 under conditions where RANTES was barely effective (71). Subsequently studies demon-strated that AOP-RANTES induced CCR5 internali-zation and inhibited recycling (48). Additionally, AOP-RANTES also inhibits HIV-1 binding to CCR3. In addition to AOP-RANTES, two modified RANTES, NNY-RANTES and PSC-RANTES have potent anti-HIV activity. The RANTES analogues showed the same rank order (PSC > NNY > AOP) in their capacity to induce prolonged CCR5 internalization, inhibit surface re-expression, and prevent HIV-1 infection (61). However, NNY-RANTES was shown to select for coreceptor switch to CXCR4-using viral variants in vivo in the hu-PBL-SCID mouse system (54). AOP-RANTES and PSC-RANTES are being tested as microbicides (34, 35, 41).
Thus far, several other small molecules have been reported to inhibit R5 virus replication. The advantage of small molecule-based inhibitors is that they are unlikely to induce signalling and therefore to indirectly augment viral entry or to induce inflam-mation. The fist small molecule inhibitor against CCR5, TAK-779 (Takeda Chemical Industries, Japan), was reported to bind strongly to CCR5 and to a lesser extent CCR2b. TAK-779 inhibits HIV-1 replication at the membrane fusion stage by blocking the interaction between gp120 and CCR5 (1). CCR5 has a hydrop-hobic pocket that fits close to the membrane surface. TAK-779 fits into this pocket and may alter receptor conformation and thereby block virus infection. Alanine scanning mutagenesis of the transmembrane domains reveals that the binding site for TAK-779 on CCR5 is located near the extracellular surface of the receptor, within a cavity formed between transme-mbrane helices 1, 2, 3, and 7 (16). The orally bioavailable versions of TAK, TAK-220 and TAK-652 have shown potent antiviral activity in vitro and are currently under in vivo study (2, 77). The potent CCR5 inhibitor, SCH-C (SCH-351125), is an oxime-piperidine compound with oral bioavailability and potent anti-HIV activity, which has been tested in HIV-1-infected individuals. 25 mg SCH-C was orally administrated twice a day for 10 days. This dose was well tolerated overall and led to > 0.5 log10 reductions in viral load in 10 out of 12 patients (57, 75). The analogous of SCH, SCH-D (SCH-417690 or vicriviroc; Schering-Plough, Kenil-worth, USA) and AD101 (SCH-350581), potently inhibit HIV-1 replication in primary lymphocytes, as well as viral entry and gp 120 binding to cell lines (74, 80, 81). Other CCR5 anta-gonists under in vitro or in vivo studies include UK-427857 (maraviroc; Pfizer, Sandwich, UK), GW-873140 (aplaviroc; Glaxo-SimthKline, London, UK), CMPD167 (Merck Research Labs, San Diego, CA, USA) and 1, 3, 3, 4-tetrasubstituted pyrrolidine deri-vatives (47, 84, 88, 89).
It has been documented that blocking CCR5-mediated HIV-1 transmission by β chemokines can be enhanced by the complementary effect of anti-CCR5 antibodies (42, 43). Because cross-reactivity with other G protein coupled receptors (GPCRs) can be an important source of toxicity for small-molecule antagonists and modified chemokines, monoclonal antibodies that have exquisite specificity for CCR5 may provide an attractive mode of therapy. A broadly inhibitory monoclonal antibody, PRO-140 (PA14) potently inhibits HIV-1 entry at concentrations that do not affect CCR5's chemokine receptor activity (59, 79). PRO140 does not induce signalling or down-regulation of CCR5. Its antiviral effect is probably exerted through a mechanism involving receptor blockade.
-
The viral component involved in the interaction of HIV-1 with CXCR4 is the V3 loop of the viral Env gp120. The natural ligand for CXCR4, SDF-1, is highly basic with overall charge of +8, just like the V3 loop of gp120, while the corresponding net charge of the extracellular regions of CXCR4 is-9. Several cationic, arginine-rich peptides, ALX40-4C (N-α-acetyl-nona-D-arginine), T-22 and its shortened version T-140 and T-134, bind to CXCR4 and prevent X4 virus infection (15, 56, 78). The small molecule, AMD3100, a bicyclam derivative, binds and antag-onizes signalling via CXCR4. AMD3100 not only strongly inhibits X4 HIV-1 replication but also blocks cell-surface-expressed HIV-1-Env-induced apoptosis of uninfected cells (14, 66). Its analogue AMD3465 is 10-fold more effective as a CXCR4 antagonist alt-hough oral bioavailability is not yet achieved (21). Nevertheless, the derivative AMD070 (AMD11070) shows good oral bioavailability and tolerance (73). KRH-1636 is another CXCR4 inhibitor (30). KRH-1636 can be absorbed through the duodenum and blocks infection of primary cells by primary and laboratory-adapted X4 viruses with similar efficiency to those of AMD3100 (30). Although CXCR4 antagonists have potential for AIDS treatment, challenges to therapies targeting CXCR4 include the wide tissue distribution of CXCR4 and the low prevalence of patients having only X4 virus.
In addition to CCR5 or CXCR4 specific drugs, development of novel inhibitors targeting both CCR5 and CXCR4 is not impossible. For instance, the viral chemokine vMIP2 can antagonize CCR5 and CXCR4 and hence blocks the infection of both R5 and X4 viruses (5, 38). A recently generated CCR2 antibody, which induces oligomerization of CCR2 with CCR5 or CXCR4, can prevent the infection of HIV-1 through CCR5 and CXCR4 (63).
CCR5 antagonists
CXCR4 antagonists
-
The most widely accepted model describing HIV-1 Env-mediated membrane fusion postulates that CD4 or/and coreceptor binding results in the formation of a coiled-coil in gp41 (7, 90). It induces the exposure of the gp41 fusion peptide, the formation of the six-helix bundle and ultimately membrane fusion. Env regions that are targets for antiviral agents are transiently exposed during the course of fusion. Several synthetic peptides derived from the first helical region (HR1 or NHR) and the second helical region (HR2 or CHR), termed as N-and C-peptides, have anti-fusion activities (19, 32). By far, the most potent fusion inhibitors, including T20 and C34, are C-peptides. T20 (originally called DP178), a 36-mer peptide base on HR2 in gp41, was previously believed to bind to the exposed grooves on the surface of the triple stranded coiled-coil, preventing the formation of six-helix bundle and membrane fusion (36). Of interest, recent studies indicate that T20 inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120 (45, 46). T20 is now a licensed drug (enfuvirtide or fuzeon; Trimeris, Morrisville, NC, USA] (39, 40). T1249, consisting of 39 L-amino acids, is a second generation HR2 peptide mimetic that combines the virtues of T-20 with sequence changes intended to increase its potency and breadth of action. C34, also based on gp41 HR2, was identified by the protein dissection method and comprises 34 amino acids spanning gp41 residues 628-661. It is soluble in aqueous solution and is a highly effective inhibitor of HIV-1 entry and cell-cell fusion (6). More recently, Sia et al has designed a potent, conformationally stable, anti-HIV-1 peptide based on the liner peptide C34, named C34 coil, using protein grafting techniques (70). A converse inhibition strategy that targets the C-terminal heptad repeat region has been employed by Root et al (64). This protein, denoted 5-Helix, comprises the six-helix bundle lacking one C-terminal peptide. The 5-Helix protein binds to the C-peptide region of gp41 and displays potent (nanomolar) inhibitory activity against diverse HIV-1 variants (64). More recently, C52L, a bacterially expressed recombinant peptide inhibitor that includes both the C-peptide and tryptophan-rich region, has demonstrated potent anti-HIV activity in both in vitro and in vitro studies (13, 85). Because C52L can be expressed in bacteria, it might be more economical to manufacture on a large scale than T-20-like peptides produced by chemical synthesis (13). Of great interest, the discovery of a natural HIV-1 fusion inhibitor from human blood, designated as VIRIP (virus-inhibitory peptide), may lead to the development of a new class of antiretroviral drugs (55). Apart from peptide fusion inhibitors, another non-peptide fusion inhibitor, designated ADS-J1, is a phenylazo-naphthalene sulfonic acid derivative, and was found to inhibit the formation of the fusion-driving six-helix bundle and HIV-1 Env-mediated membrane fusion (32).
-
In addition to being used therapeutically, entry inhibitors could be used prophylactically to prevent HIV transmission. Newly acquired HIV-1 infections are largely the result of heterosexual contact. World-wide, women face a growing risk of infection with HIV-1 (83). In the absence of an effective vaccine, mechanical barriers such as condoms can be effective in preventing sexual transmission of HIV-1. However, this method is not always accepted by male partners or is impractical (e.g., contraceptive) for use by woman. Therefore, there is an urgent need for additional interventions to prevent new HIV-1 infections. Vaginal microbicides may represent an additional important method, under the personal control of women, that can be used for the prevention of HIV-1 as well as other sexually transmitted diseases (17, 51, 68).
To prevent HIV-1 transmission, a microbicide must inactivate the virus (both free and cell-associated) while it is still in the vaginal lumen, prevent the virus from attaching to and fusion with host cells, or prevent the virus from replicating in susceptible cells. Ideally, these topical microbicides should be inex-pensive, easy to use, stable under low pH conditions, colourless, tasteless, and nonirritating to genital mucosal tissues (72, 82). To date, three microbicides tested in phase Ⅲ trials, nonoxynol-9 (N9), Savvy gel and cellulose sulphate, all belong to a'first generation' of products that aim to make the vagina less hospitable to HIV, but the results from these trials were disappointing. For N9, the failure is considered to be related to the toxicity of N9 on the vaginal mucosa, as repeated exposure may lead to inflam-mation and epithelial disruption, which may attract CD4+ T cells and facilitate HIV-1 infection (18, 91). Therefore, non-toxic and specific inhibitors, including those already in clinical trial as new anti-HIV drugs (57, 93), might be more suitable for long-term use intravaginally without destroying the integrity of the epithelium barrier. The virus attachment and fusion stages of the HIV-1 life cycle provide attractive targets for the development of mechanism-based, HIV-1-specfic microbicides. Because the mechanism of HIV-1 entry is the same for all the genetic subtypes, specific inhibitors of HIV-1 attachment, fusion and entry could prevent or hinder vaginal or rectal transmission of HIV-1, when properly formulated and applied to women or men prior to sexual intercourse (24, 53, 68, 85). Apart from specific entry/fusion inhibitors, com-pounds like carbohydrage-binding agents and poly-anions, are also potential microbicide candidates (25, 28, 68).













 DownLoad:
DownLoad: