-
Fibrinogen like protein 2 (fgl2) which encodes a serine protease is capable of directly cleaving pro-thrombin to thrombin to trigger the process of coagulation. In mice with fulminant viral hepatitis induced by murine hepatitis virus strain 3 (MHV-3) and patients with severe acute or chronic hepatitis B, it has been shown that the high expression of fgl2 results in intravascular fibrin deposition within the liver, culminating in widespread hepatocyte necrosis, and is highly correlated with disease severity (3, 10, 12). Fgl2 plays an important role in the development of MHV-3 induced fulminant hepatitis and severeacute on chronic hepatitis B in patients. The pharmacological blockage of fgl2 may offer an important new therapeutic approach in hepatitis virus induced disease.
RNA interference has proven to be an extremely potent and versatile tool to specifically reduce expression of targeted genes. Research reports demon-strate the potential for use of small interfering RNAs (siRNAs) as therapeutic agents, especially in the areas of cancer and viral infection. siRNAs can be surprisingly effecient, for example, transfections done using subnanomolar concentrations of RNA some-times achieve 90% reduction in mRNA levels (7). In this paper, a expression plasmid containing short hairpin RNA (shRNA) of mouse fgl2 (mfgl2) was constructed and its interfering effect was investigated in vitro.
HTML
-
Mouse U6 promoter (U6P) was amplified from genomic DNA extracted from mouse liver. The upstream primer (primer 1) was 5'-GTAGGATCCATC CGACGCCGCCATCTCTA-3' and the downstream primer (primer 2) was 5'-GGCAGCCAAGCTTCACA AACAAGGCTTTTCTCCAA-3'. The boldface, under-lined sequences correspond to the restriction enzyme sites for BamH I and Hind Ⅲ, respectively. The amp-lified fragment was cloned to T-vector pMD-18 (Invitr-ogen Life Technologies, Carlsbad, USA) to construct pMD-18-U6. A 19 bp oligonucleotide (5'-GCAGTG GACAGTCTGAAGA-3', S1) in the exon1 of mfgl2 and its inverted repeat (5'-TCTTCAGACTGTCCA CTGC-3', S2) formed the double strand of the constructed shRNA. The constructed shRNA was 52 bp and its sequence was 5'-GCAGTGGACAGTCTG AAGATTCAAGAGATCTTCAGAGTGTCCACTGC TTTTT-3' (S1+TTCAAGAGA+S2+TTTTT), which was produced by 3 rounds of PCR with following primers:
primer 3: 5'-CTTCAGACTGTCCACTGCAAACAA GGCTTTTCTCC-3',
primer 4: 5'-AGTCTGAAGATCTCTTGAATCTTCA GACTGTCCACTGC-3',
primer 5: 5'-AAGCTTAAAAAGCAGTGGACAGTC TGAAGATCTCTTGAAT-3' (Fig.1).
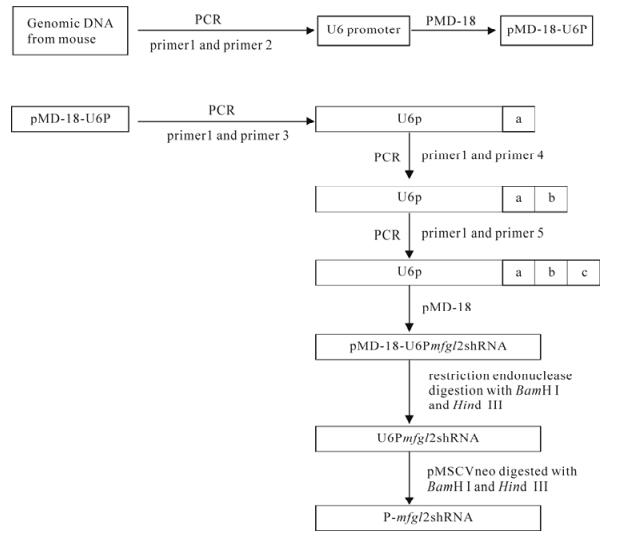
Figure 1. Flow chart of the construction of the p-mfgl2shRNA. The sequence of constructed mfgl2shRNA is 5'-GCAGTGGACAGT CTGAAGATTC –AAGAGATCTT-CAGAGTGTCCACTGCTTTTT-3'. a, b and c were one of the three parts of mfgl2shRNA from 5'-to 3', respectively.
By using the transfect vector pMD-18, U6P and shRNA template were subcloned into pMSCVneo vector (Invitrogen Life Technologies) at the BamH I and Hind Ⅲ restriction sites to construct the plasmid named p-mfgl2shRNA. By using pMSCVneo vector, a plasmid containing a fragment of the mutated shRNA acting as the experimental control was created (5'-GCAGTGGACACTCAGAGGATTCAAGAGAT CCTCTGAGTGTCCACTGCTTTTT-3'. Where the underlined nucleotides correspond to mutations with respect to the mfgl2shRNA) sequence.
-
A 1.3 kb fragment of mfgl2 was amplified (primer: 5ˊ-TCGAAGCTTGCCGCCACCATGGAGCTTCC TGGTG-3ˊand 5ˊ-TCGGATCCGTGGCTTGAAA TTCTTGG-3ˊ) from pcDNA3.1-mfgl2 (constructed by our lab) and cloned into pEGFP-N2 (Clontech Company, USA) upstream of the GFP gene at the BamH I and Hind Ⅲ restriction sites. CHO and Hela cell lines were individually cultured in 24-well or 6-well plates until 70%-80% confluence. The mfgl2shRNA plasmid (1.33μg for 6-well plates and 0.33μg for 24-well plates in each well) was mixed with pEGFP-mfgl2 or pcDNA3.1-mfgl2 (2.67μg for 6-well plates and 0.67μg for 24-well plates in each well) in serum-free F12-DMEM, and mixed with Lipofectamine (2 μg/μL, Invitrogen Life Technologies) according to the manufacture's protocol. The mutated shRNA plasmid was used as a control. After incubation at room temperature for 30 min, the mixture was added into CHO or Hela cells, and transfection was performed at 37℃ with 5% CO2. Medium was replaced with fresh complete medium 5 h after transfection. At 48h post-transfection, cells were harvested for different assays. There were 4 groups which included the p-mfgl2-shRNA group, the mutated shRNA plasmid group, a no treatment group and a blank control.
-
The expression of mfgl2-EGFP fusion protein was observed with a inverted fluorescent microscope. Positive rate of green fluorescent cells (α) was tested by FACS. 10 000 cells were counted per group. Inhi-bition efficacy=(α of no treatment group-α of p-mfgl2-shRNA group)/ α of no treatment group×100%.
-
Total RNA was extracted from mfgl2shRNA and pcDNA3.1-mfgl2 plasmid transfected CHO cells or Hela cells using Trizol reagent (Invitrogen Life Technologies) according to the manufacture's standard protocol at 48h post transfection. 1μg RNA was used for reverse transcription according to the manu-facture's standard protocol and 2 μL cDNA was amplified through PCR. The upstream primer was 5'-ACTGTGACATGGAGACCATG-3', and the down-stream primer was 5'-TCCTTACTCTTGGTCAGAA G-3'. GAPDH was used as an internal reference.
-
Cultured cell slices were blocked with 10% normal goat serum in PBS at room temperature for 2h. A polyclonal Ab to mfgl2 prothrombinase was produced in rabbits by repeated injections with a 14-amino-acid hydrophilic peptide (CKLQADDHRDPGGN) from exon 1 of the mfgl2 prothrombinase, which had been coupled to keyhole limpet hemocyanin. Ab was purified by affinity columns, and cultured cell slices were incubated with Ab (20μg/mL in PBS) at room temperature for 2h. Subsequently, slices were in-cubated with immunoperoxidase-conjugated goat IgG (6.7μg/mL) fraction to rabbit IgG Fc (Dako Cytomation) at room temperature for 1h, following which they were washed five times in PBS with 0.05% Tween20. Cultured cell slices were then air dried and photographed with a microscope.
-
Quantitative data were expressed as means ± SD. Statistical analysis was carried out by using a one-way analysis of variance, and a P value of less than 0.05 was considered statistically significant.
Construction of p-mfgl2shRNA plasmid
Transfection
Measuring the expression of EGFP
RT-PCR
Immunohistochemical staining
Statistical analysis
-
U6P was amplified from genomic DNA extracted from mouse liver. PCR products containing U6P and mfgl2shRNA are shown in Fig. 2A. The p-mfgl2-shRNA plasmid was successfully constructed as evidenced by the restriction enzyme mapping data (Fig.2B) and further confirmed by sequence analysis.
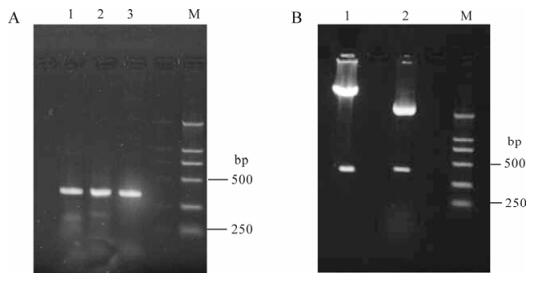
Figure 2. Construction and identification of mfgl2 shRNA plasmid. A: PCR products containing U6P plus entire mfgl2 shRNA template (a+b+c, lane 1), U6P plus part of mfgl2 shRNA template (a+b, lane 2) and U6P plus shorter part of mfgl2 shRNA template (a, lane 3). B: Restriction endonuclease analysis of p-mfgl2shRNA (lane 1) and pMD18-U6P-mfgl2shRNA(lane 2) with BamH I and Hind Ⅲ.
-
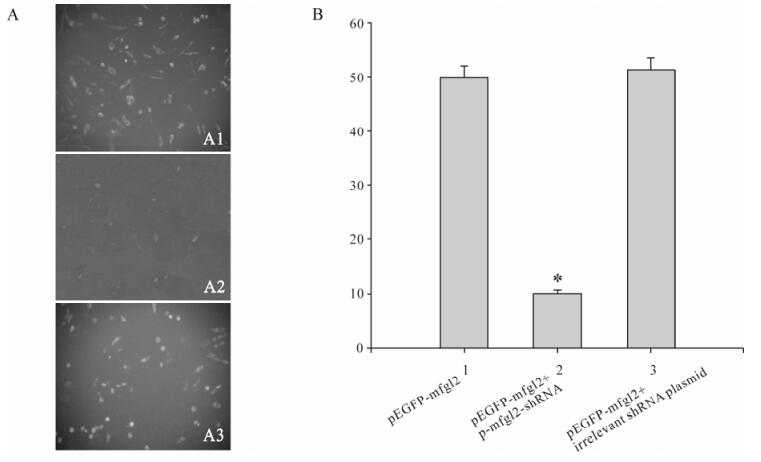
The number of green fluorescent cells and their fluorescent intensity in cells treated with p-mfgl2-shRNA decreased significantly when viewed under an inverted fluorescent microscope at 24 h, 48 h and 72 h post-cotransfection, when compared with that in cells treated with either the irrelevant shRNA plasmid or no treatment (Fig.3A). This observation was further confirmed by FACS (Fig.3B). Inhibition efficacy of the p-mfgl2shRNA group was 80.1%. There was no significant difference between the pEGFP-mfgl2 group and the mutated shRNA plasmid plus pEGFP-mfgl2 group.
-
p-mfgl2shRNA plasmid inhibited the expression of mfgl2 not only at the mRNA level as shown by RT-PCR (Fig.4A) but also at the protein level as shown by immunohistochemistry staining (Fig.4B) in both CHO cell and Hela cells. There was no significant difference between the pcDNA3.1-mfgl2 group and the mutated shRNA plasmid plus pcDNA 3.1-mfgl2 group.
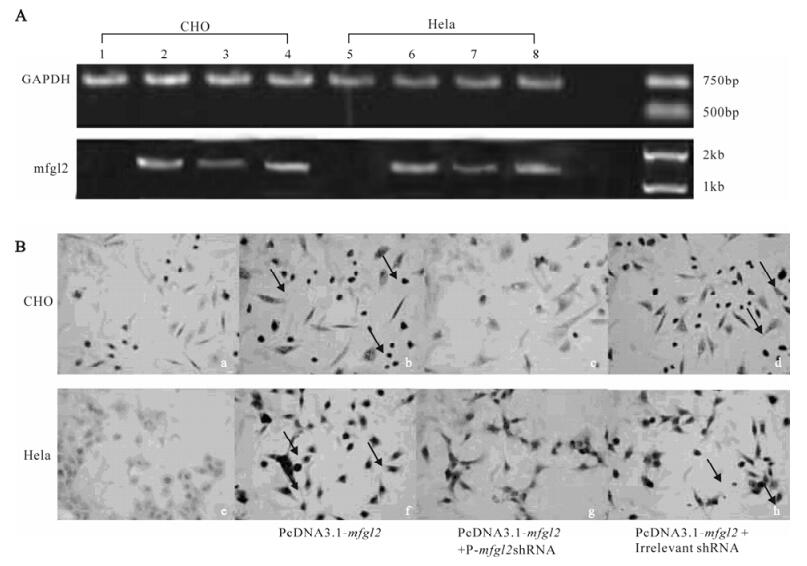
Figure 4. Effect of p-mfgl2shRNA on the expression of mfgl2 in CHO and HeLa cells. A: RT-PCR results of the transfections of pcDNA3.1-mfgl2 (lane 2 and lane 6), pcDNA3.1-mfgl2 plus p-mfgl2shRNA (lane 3 and lane 7), and pcDNA3.1-mfgl2 plus mutated shRNA plasmid (lane 4 and lane 8). The untransfected CHO (lane1) and HeLa (lane5) cells are shown as controls.B: Immunohistochemistry analysis of mfgl2.
Construction of p-mfgl2shRNA plasmid
p-mfgl2shRNA inhibited the expression of mfgl2-EGFP fusion protein
p-mfgl2shRNA plasmid inhibited the expression of mfgl2 significantly
-
Worldwide about 400 million people have chronic infection of hepatitis B virus (HBV). With a 10%-20% HBV chronic carrier rate in China, safe and effective antiviral treatments are availabel but are still far from ideal. In the Far East, fulminant hepatic failure is mainly due to viral hepatitis. The mortality of fulminant viral hepatitis is over 80% in absence of immediate liver transplantation. Recently there has been significant interest in the use of gene therapy on fulminant viral hepatitis. Nakayama et al. used adenovirus encoding immunoglobin against CTLA-4 to suppress liver injury by inhibiting acquired immune responses in a mouse model of fulminant hepatitis induced by injection of Propionibacterium acnes and lipopolysaccharide (11). Arvelo et al reported that adenoviral mediated hepatic expression of A20, an anti-apoptotic protein, protected Balb/cJ mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis, which yielded an 85% survival rate com-pared with 15%-20% in control mice (2). Song et al used RNA interference targeting Fas to elevate the sur-vival rate in mice with fulminant hepatitis and protect mice from fulminant hepatitis (15). Hecht et al. reported that a designed human IL-6/sIL-6R fusion protein promoted liver regeneration and reversed severe hepatocellular injury (4).
Our previous reports both in an experimental animal model of fulminant viral hepatitis caused by MHV-3 and patients with acute on chronic hepatitis B, have shown a critical role for fgl2 prothrombinase in the pathogenesis of fulminant viral hepatitis. Fgl2 prothrombinase belongs to the fibrinogen family of proteins and encodes a serine protease, which is an immune coagulant with the ability of directly cleaving prothrombin to thrombin in the absence of factor Ⅶ or factor X (8), resulting in intravascular fibrin deposition within the liver and culminating in widespread hepatocyte necrosis (8, 9, 10). The importance of mfgl2 prothrombinase in the patho-genesis of fulminant viral hepatitis is supported by the observation that a neutralizing antibody against mfgl2 prevents both fibrin deposition and death from MHV-3 infection (9). Furthermore, recent studies have shown that inhibition of reticuloendothelial cell mfgl2 expression through the use of gene-targeted fgl2-deficient mice results in the prevention of MHV-3 induced fibrin deposition, liver injury, and death (10). The human and murine genes for the fgl2 have been localized to chromosome 7 and 5, respectively. The murine and human proteins share 78% overall identity, with greater conservation at the C terminus. These studies argue that the fgl2 prothrombinase is a logical target for therapeutic intervention in an attempt to ameliorate fulminant viral hepatitis.
We have recently successfully constructed a mfgl2 antisense plasmid and therapeutic effects were achieved, which significantly ameliorated inflam-matory infiltration, fibrin deposition and hepatocyte necrosis, prolonged the survival time period, and elevated the survival rate in Balb/cJ mice with MHV-3 induced fulminant hepatitis (17). Antisense techno-logy has failed to gain widespread acceptance as a gene knockdown tool, largely due to the extensive experimental testing that often needs to be done to find effective target sites within the gene of interest, while it is now possible to obtain potent RNAi reagents without the extensive testing needed (1, 5, 6, 13, 14, 16). We successfully constructed a hairpin small interference RNA complementary to the sequence containing Ser89 of mfgl2 and data showed its significant inhibitory effect on mfgl2 expression in vitro. By 3 rounds of PCR a mfgl2shRNA fragment was obtained, which was convenient and avoided the difficulty of cloning a shorter DNA fragment. The recombinant vector of pEGFP-mfgl2 provides a direct and simplified methodology for primary assessment of the effect of mfgl2 siRNA on the mfgl2 gene expression. FACS showed the inhibition efficacy was as high as 80.1%. p-mfgl2shRNA plasmid inhibited the expression of mfgl2 significantly not only at the mRNA level but also at the protein level. This provides a foundation for further investigation for the application of these constructs in vivo and furthermore as a therapeutic strategy for a targeting intervention in the control of diseases to which the gene fgl2 contributes.













 DownLoad:
DownLoad: