HTML
-
Insects, as a group, are known to be infected by a range of both DNA and RNA viruses (18). Amongst these, the Baculoviridae, Poxviridae, Parvoviridae, and Reoviridae cause most of the known viral diseases in Lepidoptera. The two genera within the Baculoviridae, Granulovirus (GV) and Nucleopolyhedrovirus (NPV), have attracted the most interest as potential agents for use in the suppression of forest insect pest populations because (ⅰ) they are restricted to insects, (ⅱ) they tend to be host specific, and (ⅲ) many are known to cause epizootics within host populations. For example, population crashes due to NPV epidemics occur in many species of sawflies (Hymenoptera: Symphyta). Here, NPV infection is density dependent, and these insects can be particularly susceptible to the communication of the disease as many are communal and feed openly on foliage (Table 1). Attempts to use NPVs to suppress sawfly populations have usually met with success (7, 40). Similarly, in forest Lepidoptera, successful use of NPVs in pest suppression has been achieved with insects that feed openly on foliage and where NPV epizootics occur in a density-dependent fashion (Table 2). Examples where some degree of population suppression has been achieved through the application of NPVs are the Douglas fir tussock moth (Orgyia pseudootsugata), the white-marked tussock moth (O. leucostigma), and the gypsy moth (Lymantria dispar) (7, 33).

Table 2. Application and efficacy of nucleopolyhedroviruses against lepidopteran larvae (7)
Baculoviruses are double-stranded DNA viruses with circular genomes that range in size from 84 kilobases (kb) to over 160 kb (26). Genes are expressed in a transcriptional cascade where each successive phase depends on the successful expression of genes during the previous phase (6, 14, 15). Until recently, all fully sequenced baculovirus genomes, with the exception of the Culex nigripalpus (Diptera) NPV (CuniNPV) (1), had been from Lepidoptera (17). Sawfly NPVs (NeleNPV from Neodiprion lecontei (22), NeseNPV from N. sertifer (12), and NeabNPV from N. abietis (8), all Diprionidae] have the smallest genomes (≈82-86 kb and 89~93 open reading frames (ORFs)) and lowest G+C content (≈34%) of any published baculovirus genome. Baculoviruses typically have a conserved gene order within genera where Lepidoptera-infecting GVs show less parity to lepiodpteran NPVs than to other GVs. Hymenoptera-infecting NPVs, however, show little parity with lepidopteran NPVs or GVs, and even less with the dipteran baculovirus CuniNPV (22). Parity between sawfly baculoviruses, however, implies that there is a strong evolutionary relationship between them (8, 21). Sawfly baculoviruses clearly represent a distinct clade that diverged more recently than Diptera-infecting CuniNPV, but before Lepidoptera-infecting NPVs and GVs (12, 22).
Baculoviruses are transmitted through ingestion by a suitable host larva. In NPVs, virions are ingested as inclusions within polyhedrin protein occlusion bodies (OBs). In the alkaline environment of the larval insect midgut lumen (pH > 10), OBs dissolve, thereby releasing virions to infect midgut epithelial cells (13, 19, 11). In lepidopteran NPVs, the virus goes through an initial phase of replication in the midgut epithelium and produces singly enveloped virions that bud out (budded virus or BV) of the midgut cells to initiate the second round of infection that takes place in cells and tissues within the host hemocoel, such as hemocytes, tracheal cells, and fat body (39, 9). By the time these other cells and tissues have been infected, the infected midgut cells have been replaced by healthy cells so that the midgut becomes cured of infection. At later times following infection, the virions that are produced in the nuclei of fat body and other cells within the hemocoel are enveloped and occluded into OBs. The virions in OBs are referred to as occlusion-derived virions or ODVs. Infection of the midgut with subsequent curing as other tissues become infected results in continued feeding by affected larvae and death may not occur until 2-3 weeks after initial infection of the midgut. When lepidopteran larvae die from NPV infection, they often consist of little more than exoskeletons filled with OBs (109 to 1010 OBs per late instar larva) (39). The viral expression of cathepsin and chitinase ensures the release of the OBs in the environment following the disintegration of the larval cadavers (20, 16).
Sawfly NPVs, on the other hand, only infect the midgut epithelium so that, following initial viral replicative cycles, infected midgut cells containing OBs, are sloughed off into the frass and out of the body where they can infect other host insects (10). Death normally occurs within 1-2 weeks but, during that time, the host is producing infective units of the disease. It is likely that the continuous produc- tion and excretion of OBs from the infected host, along with the gregarious feeding habits of sawfly larvae, allows for sawfly NPV application rates, in control programs, that are two to three orders of magnitude lower than those of lepidopteran NPVs (Table 1 and Table 2).
-
The balsam fir sawfly (Neodiprion abietis) is native to and has been an occasional pest on balsam fir (Abies balsamea) in the Canadian province of Newfoundland and Labrador (NL). Since the early 1990s, it has become more important as a pest of young and semi-mature balsam fir, particularly in precommercially thinned stands (PCTs) (28). Thinning is a widely practised silvicultural technique, where the number of tree stems per hectare (ha) is reduced and spacing between trees increased by cutting down unwanted trees. Typically, balsam fir is allowed to regenerate naturally after harvesting, and immature stands are thinned when the trees are 3-5 m in height. Balsam fir sawflies overwinter as eggs laid in current-year balsam fir needles. Larvae hatch in lateJune to mid-July, depending on the weather, and feed on previous-year and older foliage for a number of weeks before pupating. Adult sawflies emerge in August; they mate and then lay eggs into September (5). Historically, outbreaks have been short in duration (3-4 years) and have been terminated by natural factors, including diseases, parasites, and predators, but predo-minantly by the balsam fir sawfly NPV (NeabNPV) (26).
Young larvae feed gregariously (4) and the later instars are responsible for most of the defoliation that occurs on foliage that is 1 year old and older (32, 34, 35). Removal of older foliage results in reduced size of current-year needles and a reduction in the number of needle primordia in developing buds (23). Loss of the older foliage, and the effect on current year foliage, reduces the photosynthesizing biomass, resulting in reduced incremental growth. Recovery of growth following severe defoliation can be slow, largely because only the older foliage is eaten (34, 35, 37). Destruction of buds in balsam fir, as happens with feeding by spruce budworm (Choristoneura fumiferana, Lepidoptera: Tortricidae) larvae, will stimulate the release of suppressed buds and increase foliar biomass (36). However, this does not happen with balsam fir sawflies because the larvae do not eat current-year foliage (32, 34, 37). Thinning appears to increase the overall severity of balsam fir sawfly defoliation in PCT balsam fir stands because balsam fir sawfly population densities are greater in thinned stands (30). Higher population densities may be due to higher rates of survival of larvae caused by a reduction in mortality associated with NeabNPV and the host plant in thinned stands (29). After defoliation has ceased, there may be a 13 to 18-year period of reduced growth before the trees recover to pre-infestation growth rates (37).
The current infestation in western Newfoundland was first detected in 1991 near Bottom Brook, east of Stephenville. Defoliation reached 1216 ha in 1994, and by 1995, moderate and severe defoliation was mapped on 12 600 ha. In 1996, the area defoliated expanded to 19 700 ha with 15 400 ha in the moderate and severe categories. In total, 53 000 ha were defoliated in 1997 with 30 300 ha in the moderate and severe categories. By 2002–2003, moderate to severe defoliation had reached 60 000 ha in western and southern Newfoundland. The western area of balsam fir sawfly infestation was of particular concern because a significant proportion consisted of balsam fir PCT stands that had been established at an average cost of $1000+/ha (total in excess of $10 million). These areas are critical to the wood supply for the local forest industries. Since the balsam fir sawfly outbreak began, the province of Newfoundland and Labrador has lost in excess of 2 m3 of growth/ha/year, a total loss in excess of 400 000 m3 of incre-mental growth (3).
-
The isolate of NeabNPV used to develop and register the commercial product AbietivTM (24, 25) was first collected from a population of balsam fir sawfly larvae south of Corner Brook (lat. 48° 57'N, long. 57° 57'W) NL in 1997 (31). Initial amplification of NeabNPV was done in the laboratory. As there are no in vitro systems for sawfly NPV production or artificial diets for sawfly rearing, all balsam fir sawfly larval rearings were done on clean, fresh balsam fir foliage that was artificially contaminated with NeabNPV by misting the foliage with an aqueous suspension of NeabNPV (106 OBs/mL). In July 1999, the total laboratory production of NeabNPV (3.3×109 OBs) was applied by helicopter, in 50 L of 20% aqueous molasses, on 2-3 ha of balsam fir sawflyinfested balsam fir forest. This field production resulted in sufficient NeabNPV to treat 1800 ha at a rate of 1×109 OBs/ha. Since 2000, NeabNPV field production has been done using fixed-wing aircraft. Each autumn, balsam fir stands across Newfoundland, but particularly in the west of the island, are routinely sampled by the Newfoundland and Labrador Department of Natural Resources to determine balsam fir sawfly egg densities to predict defoliation in the following year. Using these data, stands with egg densities averaging between 500–2000 eggs per 45-cm balsam fir branch were selected for NeabNPV production. An application rate of 3×109 OBs/ha in 2.5 L 20% aqueous molasses has been targeted against balsam fir sawfly larvae at roughly the third instar. Each year, from 2000 through 2006, 20-75 ha of forest were treated for NeabNPV production. Beginning 1 week after NeabNPV production applications, and continuing for the next 2-3 weeks, trees in the treated areas were beaten with garden rakes and falling larvae were collected on tarpaulins placed under the trees. The collected materials were then transferred to 40-kg sugar bags, additional balsam fir foliage and NeabNPV from a hand atomizer were added, and the bags were clipped shut and placed in a building at the Canadian Forest Service (CFS), Pasadena Field Station in Pasadena, NL. Insects were kept in the sugar bags until all feeding had ceased, at which point, the balsam fir branches were removed and the remaining contents transferred to 20-kg brown paper bags, which were then stapled shut. As the contents were quite dry, the dead larvae, needles, and other materials could be stored at ambient laboratory conditions (18-22℃). Dead balsam fir larvae were separated from needles and other debris using a blower-box developed by Benoit Morin especially for this purpose (Fig. 1. A and B). Collected larvae were stored frozen (–20℃) in 50-mL centrifuge tubes (Fig. 1. C) until Neab-NPV OBs were purified and counted (Moreau et al. 2005). Suspensions of 4×109 OBs/mL of NeabNPV were divided into aliquots of 40 mL in 50-mL centrifuge tubes (Fig. 1D). Field efficacy trials primarily employed the Cessna 188 aircraft (Table 3, Fig. 1E), which has a load capacity of 400 L. For ease of appli-cation, the formulation is adapted so that one entire tube (40 mL×4×109 OBs/mL=1.6×1011OBs) is added to the hopper of the airplane (400 L 20% aqueous molasses) and, applied at a rate of 2.5 L mix/ha, yields an application rate of 1×109 NeabNPV OBs/ha over a total area of 160 ha.

Figure 1. Neab NPV production. A: Dead balsam fir sawfly larvae and needles are placed in 20-kg grocery bags (GB) for storage. The blower consists of a wooden box with bathroom exhaust fans (BF) on opposite sides. A light switch (LS) turns the blowers on or off. Needles, along with dead insects, are scooped up in a beaker (B) that has a screen on the bottom and is open at the top. The beaker is then placed in the opening (arrow) of the plexiglass funnel (PF). Because the needles are lighter than the dead balsam fir sawfly larvae, the needles blow out of the funnel, through the dryer duct (DD) and are collected in a plastic tub (PT) and discarded. When the blower is turned off, the dead insects fall back into the beaker and are placed in a separate container (not shown) for further processing. B: The staged removal of the needles and capture of the dead balsam fir sawfly larvae moving from bottom to top. The sample is progressively cleaned of balsam fir needles and other unwanted debris until mostly NeabNPV-killed balsam fir sawfly larvae remain. C: Clean larvae are stored frozen in 50-mL conical centrifuge tubes until NeabNPV is purified from them. D: Abietiv ready for application. There is sufficient NeabNPV in each of these 50-mL centrifuge tubes to treat 160 ha of balsam fir sawfly-infested forest at a rate of 1×109 NeabNPV OBs/ha in 2.5 L 20% molasses/ha. E: Forest Protection Limited Cessna 188 loaded with Abietiv and taxiing for take-off at Deer Lake Airport, NL. F: Printout of a spray operation carried out over an irregular-shaped block in western Newfoundland in 2001. The lines within the block indicate where the spray booms were on. Gaps in the lines are either water bodies, cutovers or other areas where there are few or no trees.

Table 3. Aerial field trial applications of NeabNPV against the balsam fir sawfly in western Newfoundland
-
To register a microbial control product in Canada, the Pest Management Regulatory Agency (PMRA) of Health Canada, requires that field efficacy trials be carried out (2). For the registration of NeabNPV, field trials were carried out in 2000, 2001, and 2002 (31), with supplemental trials being carried out in 2003, 2004, and 2005 (24). In total, approximately 22 500 ha were treated with NeabNPV at a rate of 1-3×109 OBs/ha in 2.5 L 20% molasses/ha (Table 3). Trials were carried out roughly between 20-30 July each year when the larval indices were between 1.5 and 3.0 (31). Sprays were done in the morning between 06:00 and 11:00 and in the evening between 17:00 and 21:00 on days when rain was not expected for 24 h. Corners of blocks were determined on the ground using global position system (GPS) coordinates, which were then used to establish the boundaries of each block. The boundary coordinates were then uploaded into the computer onboard each aircraft. Each aircraft used was equipped with an Ag-Nav®2 differential GPS navigational system (www.agnav.com). This, in turn, was linked to systems that automatically turned the spray booms on and off, regulated flow rates in response to airspeed, and recorded these and other data over the course of the spray operation. From these data, maps could be produced that showed the lines sprayed over each experimental spray block (Fig. 1.F). This was not only important to verify the accuracy of the spray operation for experimental purposes but also to ensure that restrictions placed on the operation (e.g., buffers around water bodies) were observed. The approximate locations of the NeabNPV-treatment blocks are shown in Fig. 2.
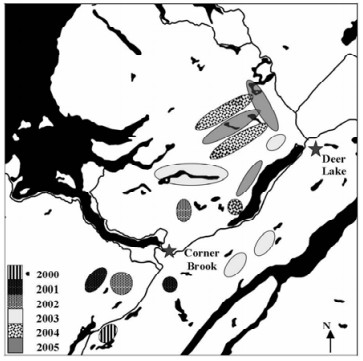
Figure 2. Approximate locations of the Abietiv field efficacy trials carried out in western Newfoundland between 2000 and 2005. The total area treated was approximately 22 500 ha.
Analysis of the data from efficacy trials carried out in 2000–2002 (31) showed that, in the weeks that followed the treatment, both levels of NeabNPV infection and frass production increased in association with larval instar. However, levels of infection increased more rapidly in treated than in control blocks. In parallel, frass production was 31% lower in treated than in control blocks. Although some differences were detected between treated and control blocks for the speed of population decline following the aerial spray in each trial, results were not consistent, with treated populations sometimes declining faster, at the same speed, or slower than control populations. Depending on the rate of change of populations, variable results with respect to insect density were observed in the year following the aerial spray. With increasing populations, as in 2000 (positive rate of change in control blocks), egg-to-third-instar density was almost one order of magnitude lower (10-fold difference in density) in treated than in control blocks in the year following the NeabNPV application. With peaking populations, as in 2001 (rate of change close to zero in the control block), egg-to-third-instar density was half an order of magnitude lower in the treated than in the control block in the following year. With decreasing populations, as in 2002 (negative rate of change in control blocks), egg-to-third-instar density was similar in treated and control blocks. The study by Moreau et al. suggests that increasing or peaking population outbreaks of the balsam fir sawfly are optimally and successfully suppressed by aerial applications of NeabNPV at rates as low as 1×109 OBs/ha (31).
-
Much of the history of the registration and comerciallization of Abietiv has been described elsewhere (24, 25). Briefly, the documentation for the registration of Abietiv was submitted to the PMRA in June 2004. In January 2005, a request for additional information, data, and clarification was received by the registrant (CJL for CFS) and this information was forwarded to the PMRA in April 2005. Additional information was requested in August 2005 and, as the amount of information required was minimal, this was supplied within 2 weeks. Abietiv received conditional registration in April 2006. Conditional registration only was granted because additional studies on product shelf life were required. However, the conditional registration status did not hinder the sale or operational application of Abietiv.
In May 2005, CFS signed a licensing agreement with Forest Protection Limited (FPL) (www. forestprotectionlimited.com) for the commercialization and sale of Abietiv. For the purposes of development of Abietiv and other baculoviruses for use in forestry, FPL along with BioAtlantech Inc. (www.bioatlant ech.nb.ca) formed the company Sylvar Technologies Inc. (www.sylvar.ca), which was incorporated on 28 June 2006. On 1 July 2006, Sylvar Technologies delivered a supply of Abietiv to the Newfoundland and Labrador Department of Natural Resources, who applied it to 15 000 ha of balsam fir sawfly-infested forest in western Newfoundland.














 DownLoad:
DownLoad: