-
Hz-2V (a.k.a. gonad specific virus, GSV) is unusual among insect pathogenic viruses in that productive replication of this virus does not lead to insect mortality but rather to the sterility of the infected adult moth. This fact is the principle feature of the virus that led to its discovery in a laboratory colony of Helicoverpa zea from Stoneville, MS (6, 11). The Stoneville colony of H. zea, later found to be carrying the virus, was difficult to maintain and periodically went through periods of low fecundity due the high level of sterile individuals in the colony (7). It was later determined that these sterile moths had malformed reproductive tissues, a condition which has been referred to as being agonadal (AG).
Insects from the Stoneville colony were found to be able to transmit Hz-2V to healthy partners during mating as evidenced by the AG progeny resulting from these matings (6). In a series of experiments involving matings between infected, Stoneville insects and healthy partners, it was demonstrated that not only was Hz-2V transmitted by both infected males and females during mating, but also that some Stoneville females were fertile, asymptomatic (AS) carriers of the virus. These AS individuals gave rise to AG progeny when mated to healthy male moths. In addition, the results of these mating experiments suggested that only females could be AS carriers of the virus, since individual mating pairs of Stoneville males and healthy females produced either no viable eggs or only healthy progeny. Virus infected AG progeny were only observed to result from mass matings of Stoneville males and healthy females; presumably arising from matings of uninfected Stoneville males and females infected with Hz-2V via prior mating attempts with infected, AG Stoneville males.
Another important finding of these mating experiments was that the level of AG progeny hatching from eggs produced by matings with infected Stoneville moths increased with increasing oviposition days. This suggested that productive virus replication took place in infected, fertile females (either AS carriers or those acquiring virus from infected males) with an increase in virus dose being transferred to eggs laid on successive oviposition days. The site and basis for this virus replication, as well as the mechanism by which Hz-2V is transferred to eggs developing in infected females, has yet to be determined.
HTML
-
Hz-2V is a large, rod-shaped, nonoccluded, enveloped virus of approximately 437 nm by 93 nm (1). In infected females, enveloped virus particles can be found in vesicles, many of which are aggregated together to makeup a "sticky" mass known as the "virus" or "waxy" plug that covers the reproductive opening of these females (3) (Fig. 1 and Fig. 2). Virus particles purified from this plug have a loosely associated envelop made up of rope-like structures which run the length of the envelope, parallel to the nucleocapsid. Viral nuclecapsids are approximately 378 nm by 55 nm and have a smooth regular surface similar to that of baculovirus nucleocapsids (Fig. 3).
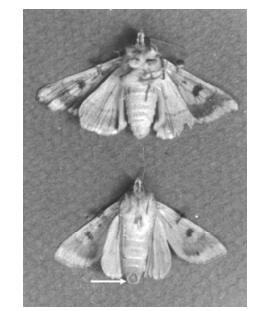
Figure 1. Healthy (top) and Hz-2V infected H. zea female moth (bottom). Arrow indicates presence of "virus plug". Reprinted from the Journal of Invertebrate Pathology, Vol 70, Burand and Lu, Replication of a gonad-specific insect virus in TN-368 cells in culture, 88-95, Copyright 1997, with permission from Elsevier.
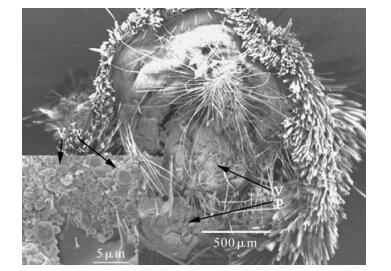
Figure 2. Scanning electron micrographs of the terminal abdominal segment of an AG female moth showing the position of the "virus plug" (Vp) covering the reproductive openings of this insect. Also shown in the insert are the round virus vesicles (Vv) that make up the "viral plug". Reprinted from the Journal of Invertebrate Pathology, Vol. 85, Burand, Rallisand Tan. Horizontal transmission of Hz-2V by virus infected Helicoverpa zea moths. 128-131, Copyright 2004, with permission from Elsevier.
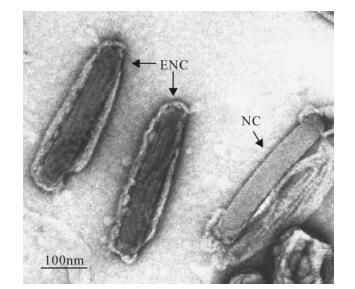
Figure 3. Electron micrograph of Hz-2V virus particle showing enveloped nucleocapsids (ENC) and a nucleocapsid from which the envelope has been partially removed (NC). Bar, 100nm. Reprinted from the Journal of Invertebrate Pathology, Vol 70, Burand and Lu, Replication of a gonad-specific insect virus in TN-368 cells in culture, 88-95, Copyright 1997, with permission from Elsevier.
The virus has a double stranded circular DNA genome, 233 kb in length, which codes for approximately 100 genes. The sequence of the Hz-2V genome is 95% identical to the sequence of the Hz-1V genome (15). Hz-1V was initially isolated from an ovarian cell line of H. zea (5) and has been shown to replicate only in cell culture with no demonstrated pathology in insects. This lack of demonstrated in vivo host pathology for Hz-1V is the primary difference between these two closely related viruses.
-
Although Hz-2V is infectious per os, the major route for the horizontal transmission of Hz-2V in nature appears to be through direct contact between insects during mating attempts (6). The virus is not stable for long periods of time outside the insect, which is a reflection of the close association that has evolved between Hz-2V and its host and the need for direct contact between insects for virus transmission. The "viral plug" is thought to play an important roll in virus transmission since healthy males making sexual contact with AG females are capable of infecting healthy females upon subsequent matings, as evidenced by the fact that healthy females mating with contaminated males give rise to infected progeny (3).
-
The reproductive tissues of Hz-2V infected male moths are grossly malformed into a "Y-shaped" structure (6, 14). These AG males have small, unfused testes and lack seminal vesicles, vasa deferentia and accessory glands (Fig. 4). The reproductive tissues in normal males responsible for producing sperm, the pheromonostatic peptide (PSP, see Behavior below) and the spermatophore, are absent or grossly malformed in infected males. In these males, the reproductive tissues essential for the initiation of copulation and the transfer of reproductive fluids to female moths during mating appear to be intact and functional. EM observations of reproductive tissues from infected males revealed the presence of large numbers of virus particles in the lumen of the primary simplex; suggesting that AG males may be able to mate with healthy female moths and infect them, without fertilizing them or altering their sexual receptivity for subsequent mating with other male moths.
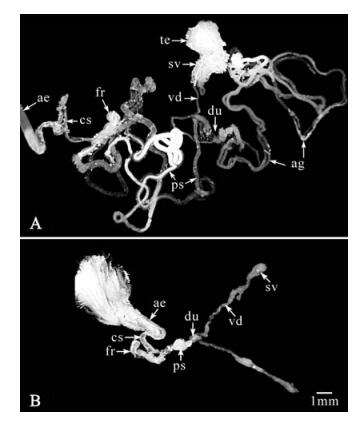
Figure 4. Reproductive tissues of adult H. zea male moths showing malformation of these tissues resulting from Hz-2V infection. (A) Normal male, accessory glands (ag), testes (te), seminal vesicles (sv), vasa deferentia (vd), duplex (du), primary simplex (ps), cuticular simplex (cs), twisted area of the cuticular simplex where the frenum of the spermatophore is formed (fr), and the aedeagus (ae). (B) Agonadal male exhibiting grossly malformed reproductive system lacking testes and accessory glands, but exhibiting other tissues corresponding to the same tissues of normal male. Reprinted from the Journal of Invertebrate Pathology, Vol 80, Rallis and Burand, Pathology and ultrastructure of the insect virus, Hz-2V, infecting agonadal male corn earworms, Helicoverpa zea, 81-89, Copyright 2002, with permission from Elsevier.
AG female moths lack ovaries, bursa copulatrix, accessory glands and spermatheca and have grossly deformed and enlarged common and lateral oviducts, which appear as a large "Y-shaped" structure (6, 13). An examination of the reproductive tissues of virus infected, female pupae 48 hr. after pupation revealed the malformation of these tissues with the absence of ovariols and the presence of this "Y-shaped" structure. In both pupae and adults the branches of the "Y-shaped" structure are much larger than the corresponding normal, lateral oviducts (Fig. 5). EM observations of the branch structure from 8-day-old, AG pupae show a significant amount of virus replication in cells that had proliferated in these tissues.

Figure 5. Reproductive systems of adult female H. zea moths showing malformation of these tissues resulting from Hz-2V infection. (A) Tissues from a normal female ovarioles (ov), lateral oviducts (lo), common oviduct (co), cervix bursa (ce), corpus bursa (cb), and seminal duct (sd). (B) Grossly malformed reproductive tissues of a typical agonadal female adult moth arising from an egg laid by an infected adult female. Note the difference in development of the lateral oviducts, common oviducts, cervix bursae, and corpus bursae in each of the panels. Reprinted from the Journal of Invertebrate Pathology, Vol 81, Rallis and Burand, Pathology and ultrastructure of the insect virus, Hz-2V, infecting agonadal female corn earworms, Helicoverpa zea. 33-44, Copyright 2002, with permission from Elsevier.
The hypertrophy of the lateral oviducts in infected females and the proliferation of the cells in these tissues suggest that the Hz-2V has reprogrammed their development to augment its own replication. It is surprising that virus replication in these tissues during their development in the last larval instars and in pupae does not destroy these tissues but rather results in cell proliferation and an actual increase in their size compared to those in healthy females.
In 10-day-old pupae, extensive virus replication was observed in cells throughout the "Y-shaped" structure and virus particles were visible in the lumen of these tissues. These virus particles appear then to move through the lumen to the bursa where they accumulate, associated with a matrix of darkly staining material. Upon emergence, the virus appears to coalesce with this material to form a large number of virus vesicles which makes up the "virus plug" covering the tip of abdomen of AG female moths (13).
-
One of the most interesting properties of Hz-2V is its ability to manipulate both the physiology and behavior of the infected host to favor viral transmission and enhance viral fitness. Infected, AG males lack accessory glands and therefore are incapable of producing PSP, a male specific peptide that when transferred to females during mating causes a dramatic drop in pheromone titer resulting in the temporary suspension of the females' mate calling behavior (8). The injection of females with extracts from the reproductive tissues of healthy males resulted in the cessation of calling by these females, while females injected with extracts from AG males continued to call (2).
The inability of virus infected AG males to block female calling likely plays an important role in virus transmission since infected males placed in close proximity to females attempt to mate and make frequent, brief contacts with calling females which continue to call even after these mating attempts (2). In the mass mating experiments (6) with healthy males discussed previously, it is likely that these brief attempts at mating by infected males resulted in infection of females which continued to call and subsequently mate with fertile males.
H. zea females may mate several times, but generally not more than once in a given night (12). This mating pattern is thought to be due to the level of sex pheromone produced by the female. The production of pheromone begins during the first scotophase following adult emergence and peaks during the second and third nights. A peak in pheromone titer triggers female mating and "calling" behavior, which includes rapid wing vibrating, the extruding of the ovipositor and release of sex pheromones via the pheromone gland. The calling behavior elicited by females results in the attraction of mates and receptivity or acceptance of males that attempt to mate. After mating, pheromone titers decline and the female looses sexual receptivity due to the transfer of male-derived anti-calling factors, the spermatophore, and other reproductive fluids including PSP (8, 9). This loss of receptivity is only temporary, as pheromone production may resume on nights subsequent to the night of mating. Since Hz-2V-infected, AG males lack accessory glands, yet have what appears to be a fully functional aedeagus and endophalus, it is possible that these sterile, infected males that do not transfer PSP infect healthy females during their attempt to mate. Without the transfer of PSP, healthy females would remain receptive after these mating attempts, and continue to call and possibly mate with healthy, fertile males during that same night. After becoming fertilized, newly infected females resemble AS females and can pass the virus on to their progeny, some of which emerge as AG adult moths.
In mating experiments with individual pairs, healthy females do not exhibit calling behavior after mating with healthy males but do continue to call after attempted matings by AG males (2). This indicates that within a single night, a healthy female could become infected by an AG male, still remain receptive and become fertilized during a subsequent mating with a healthy male. When we conducted mass mating experiments where healthy females were placed with AG males for two nights then mated with healthy males, 6 of the 55 resulting progeny insects were AG and 42 were AS. The results from these experiments demonstrate that AG males are physically capable of attempting to mate with healthy females and can infect them, yet at the same time these males are probably not capable of producing or transferring PSP during mating. These matings result in virus infected females that remain receptive to males, and subsequent matings between these females and healthy males produce not only virus-infected progeny but also newly contaminated males that can serve as vectors for further disseminating the virus. Because male corn earworms are promiscuous, both AG and virus contaminated males could potentially mate with, and infect, multiple females during a given night, favoring the spread of Hz-2V throughout the population.
We have recently shown that AG females exhibit calling and mating behavior and remain receptive to mating with healthy males (2). In our observations of the calling and mating behavior during this time, wing fanning by virus-infected females appeared normal, although the presence of the "viral plug" inhibited the full extrusion of the ovipositor in these insects.
In flight tunnel experiments (4), virus-infected females were attractive to males and when given a choice between healthy or AG females, males flew to AG females almost twice as frequently as they did to healthy females (64.63% compared to 35.37%). This difference is significant (p = 0.019) and indicates that virus-infected females are more attractive to males than normal, healthy females. The reason for the increased attractiveness of virus-infected females appears to due to the increased amount of pheromone produced by the pheromone glands of these insects. We found the total amount of pheromone produced by glands from AG females was five to sevenfold higher than that produced by glands from healthy females (p < 0.05, Kruskal-Wallis tests) (4). Since the "virus plug" covering the reproductive opening of AG females contains a high concentration of virus particles (3), one possible reason for the elevated level of pheromones and the increased attractiveness of these females could be to facilitate virus transmission to males that then could become contaminated with the virus during attempted matings.
-
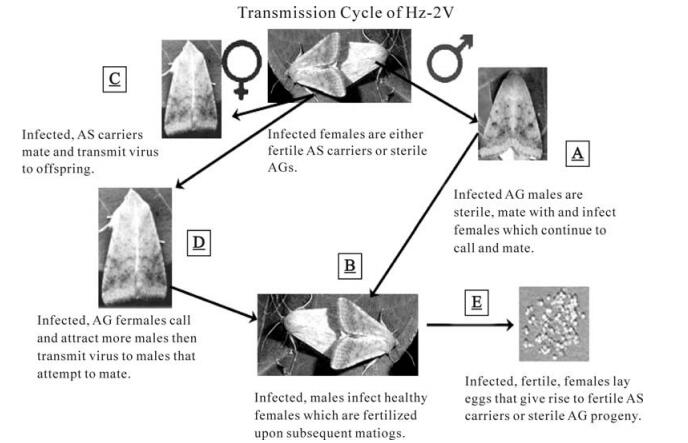
Based on what we currently know about the biology and transmission of Hz-2V, we have developed a model of the replication and transmission of the virus (Fig. 6). In this model, AG males that attempt to mate with healthy females transmit virus to these females but do not fertilize them or transfer PSP (Fig. 6A). As a result, these females become infected, and virus replication proceeds in the reproductive tissues of these individuals. These newly infected females remain receptive and after mating with a fertile male (Fig. 6B), transfer virus into eggs, which results in infected progeny that are either AG or AS carriers of Hz-2V (Fig. 6E).
In the case of fertile, AS females (or females injected with the virus), replication takes place in reproductive tissues during egg development, with the virus being transmitted to progeny insects via the egg (Fig. 6C). In the model, infected progeny insects resulting from these females appear normal as larvae and pupae, carrying the virus in a persistent state. In females that become AS, the virus remains in this persistent state, being activated during mating or egg development and then transferred to progeny insects. For insects that become AG, we propose that during the initiation of development of reproductive tissues there is a coincident expression of a specific set of viral genes that results in the proliferation and reprogramming of cells that make up these tissues. The expression of this set of viral genes then ultimately results in the malformation of these tissues and a manifestation of the AG condition in adult moths. In the case of AG females (Fig. 6D), these insects exhibit "calling" behavior, and as a consequence of virus replication, produce more pheromone than healthy females and attract more male moths. Virus replication in these AG females leads to the formation of the "virus plug", which serves both as a source of contaminating virus for healthy males that attempt to mate with these insects and acts as a "mating plug" (10) preventing the transfer of spermatophore and PSP from healthy males. As a result these females continue to call and transmit virus to other healthy males that attempt to mate with them.
Hz-2V has clearly evolved a close association with the reproductive biology of its insect host. As part of its evolution, this virus has gained control of the development of the host's reproductive tissues, reprogramming their development and causing the cells that make up these tissues to proliferate, ultimately resulting in the production of large numbers of virus particles. The ability of Hz-2V to alter and reprogram the development of these tissues suggests that in the evolution of the virus it has acquired genes that are able to regulate these processes in the insect. It is not uncommon for a virus to alter the physiology and behavior of its host to enhance transmission and increase fitness. What is unique about Hz-2V, however, is how dramatically the reproductive physiology and behavior of the infected moth is altered by this virus and that the infected moth, which is sterile as a result of virus replication, continues to be a vector for transmission of the virus. Understanding the unique biology of Hz-2V and the process by which virus replication results in the sterility of infected moths promises to provide useful molecular tools that can be applied to future development of novel insect pest control strategies.













 DownLoad:
DownLoad: