-
Ribosome inactivating proteins (RIPs) are a group of plant enzymes that inhibit polypeptide chain elongation by inactivating the ribosome [24]. All plant RIPs share the common property of inactivating ribosomes and inhibiting protein synthesis. RIPs damage ribosomes in an irreversible manner by removing adenine residue from 28S rRNA by N-glycosidase activity (Fig. 1). The removal of one adenine base renders the 60S subunit of eukaryotic ribosomes unable to bind the elongation factor 2 (EF-2), with consequent arrest of protein synthesis [23]. RIPs are classified into three types: type Ⅰ composed of a single polypeptide chain; type Ⅱ, a heterodimer consisting of an A-chain, functionally equivalent to a type Ⅰ, which is attached to a sugar-binding B chain; Type Ⅲ, a single chain containing an extended carboxyl-terminal domain with unknown function [20, 25].
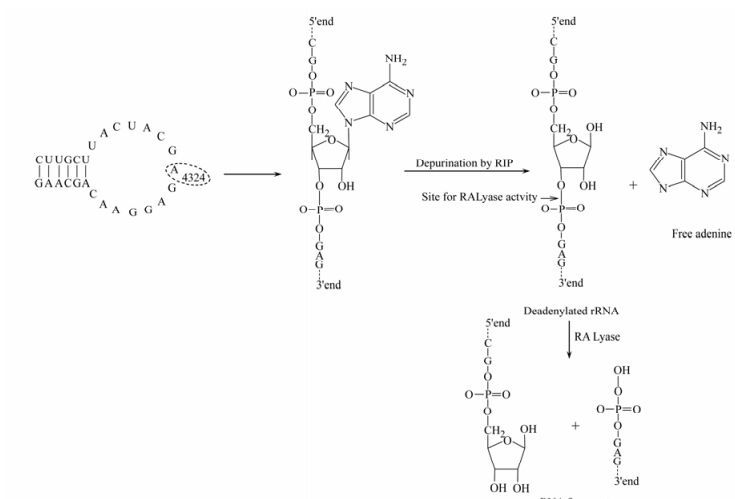
Figure 1. Mechanism of action of RIPs: A specific N-glycosidase activity cleaves adenine (A4324) which is located in sarcin/ricin loop of the ribosomal RNA of the large subunit. The reaction product of the RNA N-glycosidase (Deadenylated rRNA) acts as a substrate for 'ribosomal RNA apurinic site specific lyase' (RALyase), which cleaves the phosphodiester bond 3' end. The reaction products are a short (4325-4785) 3'-end fragment and a long (1-4324) 5'-fragment with free 3'-hydroxyl terminus [20].
Ribosome inactivating proteins (RIPs) exhibit important antiviral properties against viruses by virtue of their broad-spectrum antiviral activity. RIPs inhibit replication of RNA as well as DNA viruses. Antiviral research has been focused on RIPs that interfere with various parts of the viral life cycle and most of the recent work has been focused on the ability of RIPs to act against Human immunodeficiency virus (HIV) [11, 21]. Trichosanthin (Trichosanthes kirilowii), PAP (Poke weed americana) and MAP30 (Momordica charantia) have been reported to inhibit HIV-1 replication in vitro [1]. Trichosanthes was the first RIP to be tested in phase I clinical trials during a time when AZT (zidovudine) was the only drug approved to treat HIV infection [4]. However, the exact mechanism of antiviral activity is still not clear. It was initially thought it involved inactivation of the host cell ribosomes, leading to inhibition of viral protein translation and host cell death. However, with the help of recombinant techniques, mutated RIPs were produced and it was possible to ascertain that the ribosome-inactivating and antiviral activities could be separated [24]. The aim of this review is to describe several plant RIPs and discuss recent progress in establishing the anti-viral activities of these proteins.
RIPs have antiviral activity against plant, fungal and animal viruses. The antiviral activity against animal virus has led to numerous studies on the effect of RIPs. Investigations on RIP were started with the hope that these could be used for AIDS therapy [12, 13]. Important advances were made in the field of RIPs from plant, thereby focusing on their in vitro anti-HIV model as well as on their mechanism of action. The replication of HIV was observed to be inhibited by several RIPs, including MAP30, GAP31, PAP and trichosanthin [18].
HTML
-
Trichosanthin is a type Ⅰ RIP extracted from the root tuber of Trichosanthes kirilowii. TCS possess a wide spectrum of biological and pharmacological properties and preferentially inhibited replication of human immunodeficiency virus type Ⅰ (HIV-1) in both acutely infected T-lymphoblastoid cells and chronically infected macrophages in vitro [16]. Three TCS mutants, TCSM(120-123), TCSE160A/E189and TCSR122G were constructed to establish the relationship between anti-HIV and ribosome inactivating activity. When ribosome inactivating activity dropped 1800-fold (TCSE16A/E189A) and 4000-fold (TCSMC120-123), the mutant lost almost all of the anti-HIV activity but when ribosome inactivating activity was only 160-fold decreased (TCSR122G), the mutant still maintained some anti-HIV activity. TCSR122G (10 µg/mL) also reduced HIV p24 antigen expression by 46.4%, inhibited cell growth by 36.1% and TCS reduced HIV p24 antigen expression by 78.2% and inhibited cell growth by 43.2% at 10 µg/mL concentration. nTCS (natural TCS) anti-HIV activity is related to its ribosome inactivating activity. HIV replication inhibition was partly due to inhibition of cell proliferation by TCS and its mutants [30].
TCS prevented HIV-1 DNA integration in a dose-dependent manner in cell culture, but did not interfere in viral entry and reverse transcription, but also failed to induce cytotoxicity. HIV-1 integrase can integrate HIV-1 long terminal repeats into cellular chromosome. TCS can efficiently bind and depurinate HIV-1 DNA integration. TCS specifically binds and depurinate HIV-1 long terminal repeats, which is the substrate for HIV-1 integrase and may be responsible for the inhibitory activity on HIV-1 integration [32]. TCS can penetrate into HIV-1 virions and shows potent anti-viral activity of TCS within virions. It has a strong tendency to associate with and penetrate the viral envelope and contribute to virus-inhibition activity. When compared with free TCS, the virions carrying TCS showed a highly inhibitory effect on HIV-1 replication and the TCS in virions invade infected cytosol upon viral infection. The antiviral effect of free TCS was not so prominent when used in trace amounts but the same amount of trace TCS in virus-carrying state significantly reduced the HIV-1 infectivity [33].
-
Pokeweed antiviral protein (PAP) is a ribosome inactivating protein isolated from the leaves of Phytolacca americana and has a unique ability to depurinate HIV-1 RNA. The anti-HIV activity of PAP was attributed to its ability to inhibit viral protein synthesis and depurinate HIV-1 RNA [22]. The antiviral activity of PAP is highly cell selective and greatly enhanced by conjugation to antibodies specific for cell-surface receptors that are capable of being internalized upon ligand occupation [31]. Immunoconjugates TXU (anti CD7)-PAP and B53 (anti-CD4)-PAP inhibited viral replication in a dose dependent manner. The anti-HIV activity of TXU-PAP was slightly more potent as compared to anti-HIV agent such as AZT, d4T, unconjugated PAP and B53-PAP and was not significantly cytotoxic to T-cells. PAP-containing immunoconjugate antivirals influence both functional and structural proteins of HIV-1 without any significant cytotoxicity of T-cells. The TXU-PAP immunoconjugate elicited potent anti-HIV activity in the Hu-PBL-SCID mouse model of human AIDS without any greater side effects compared to B53 (anti-CD4)-PAP immunoconjugate. All mice treated with TXU (anti-CD7)-PAP or B53 (anti-CD4)-PAP remained healthy and no unusual response or signs of ill health were observed [28].
Two nontoxic recombinant PAPs, FLP-102 (151AA152) and FLP-105 (191AA192) were engineered and produced in E. coli and possess potent anti-HIV activity. These nontoxic recombinant PAPs depurinate HIV-1 RNA much better than rRNA and potent anti-HIV agents compared to native PAP or recombinant wild-type PAP. FLP-102 exhibited better selective anti-HIV activity and can deadenylate HIV-RNA more efficiently than wild-type PAP. FLP-102 and FLP-105 also inhibit replication of HIV-1 strain HTLV III B in human peripheral blood mononuclear cells (PBMCs). Both proteins inhibited HIV-1 replication in a dose dependent manner with an IC50s of 0.7 ± 0.2 μg/mL for FLP-105 and 0.2 ± 0.0 μg/mL for FLP-102. The nontoxic PAPs were less toxic than wild-type PAP in BALB/c mice. Both FLP-102 and FLP-105 exhibited potent in vivo activities against a nucleoside reverse transcriptase inhibitor (NRTI)-resistant clinical HIV-1 isolate in a surrogate human peripheral blood lymphocyte (Hu-PBL SCID) mouse model of human AIDS [29]. It was shown by D'cruz et al in 2001 that PAP acts as a nonspermicidal microbicide for preventing sexual transmission of viral sexually transmitted disease. Studies also showed that PAP was non toxic to both human sperm and female genital-tract epithelial cells at a concentration 2000 times higher than its IC50 value against HIV-1 and had no adverse effect on either female genital-tract epithelial cells or sperm function. PAP was also active against other sexually transmitted pathogens in semen and sperm function was preserved after sperm was pretreated with PAP as a prophylactic antiviral agent. Pretreatment of PAP had no adverse effect on sperm transport within cervical mucus or on in vitro sperm-egg binding and fusion [5].
-
MAP30 is a momordica antiviral protein isolated from seeds and fruits of Momordica charantia and is a potent inhibitor of HIV-1 infection and replication. It inhibits p24 expression and viral-reverse transcriptase (RT) activity. MAP30 showed no inhibition of cellular DNA synthesis and protein production at 33.4nM, a concentration where 98% and 87% inhibition of p24 and RT activity respectively was achieved. MAP30 exhibited dose dependent inhibition of HIV-1 infection and replication measured by: (a) quantitive focal syncytium formation on CEM monolayers; (b) viral core protein p24 expression; and (c) viral-associated reverse transriptase (RT) activity in HIV-1 infected human T-cell line cells [14].
Recombinant MAP30 exhibited similar anti-HIV ativity as observed by nMAP30 (natural MAP30). Both rec-MAP30 and nMAP30 showed dose dependent inhibition of HIV-1 replication. The ID50of both nMAP30 and rec-MAP30 were nearly identical at between 0.2nM and 0.3nM for syncytium formation, p24 inhibition and RT inactivation. Rec-MAP30 was also non toxic to uninfected H9 cells and had no effect on cellular DNA and protein synthesis [12].
MAP30 is a potent inhibitor of HIV-1 integrase which is responsible for the integration of viral DNA into the host genome. The integrated DNA serves as the template for the transcription of viral genes. The integration process requires two viral components: integrase, and specific cis-acting DNA sequences located at the ends of the viral long terminal repeats (LTRs). MAP30 exhibited dose-dependent inhibition on the strand transfer activity of HIV-1 integrase and total inhibition was observed at stoichiometric concentrations of the HIV inhibitor and HIV integrase. Inhibition was also studied by using oligonucleotide substrates with sequence corresponding to U5 regions of HIV LTR [13].
The proteolytic fragments of MAP30 found to be biologically active. During protease digestion, the central fragments of the MAP30 were protected from proteases and the N-and C-termini were more susceptible to cleavage. Three major proteolysis fragments were generated and two largest fragments retained anti-HIV activity (MW of 25-26kDa and 21-22 kDa). The central proteolytic fragment prevented topologically relaxation of supercoiled DNA, inhibiting HIV-1 integrase and HIV-1 and p24 expression. These proteolytic fragments were neither inhibitory to cell-free translation nor cytotoxic unlike the full length MAP30. The N-and C-termini of MAP30 were not necessary for anti-HIV activity but were required for ribosome inactivation. The antiviral activity of MAP30 is independent of ribosomal inactivation activity [8].
Recently, a novel ribosome-inactivating protein (RIP), balsamin was purified from the seeds of Balsam apple, Momordica balsamina. It has a molecular weight of 28 kDa by SDS-PAGE analysis. Balsamin inhibits protein synthesis in a rabbit reticulocyte lysate-based cell free translation assay with an IC50 of 90.6 ng/mL [11, 21]. Theanti-viral activity against HIV of the purified protein is under progress.
-
Hepatitis B virus is a major agent for liver diseases in Asian countries. It also plays important roles in the development of liver diseases other than HBV. Also, a significant proportion of HIV-infected patients are coinfected with hepatitis B virus (HBV) and these patients are at risk of developing HBV disease-associated outcomes, such as cirrhosis, hepatocellular carcinoma and eventually death [2]. MAP30 from Momordica charantia showed inhibition of HBV DNA replication and HBsAg secretion. The anti-HBV activity of MAP30 was studied on human hepatoma G2.2.15 (Hep G2.2.15) cells. MAP30 showed effective inhibition of HBV gene expression and genome replication of HBV DNA of intracellular replicative intermediates was decreased. MAP30 inhibited the expression of HBV antigen, decreased the viral DNA replication, downregulated replicative intermediates and weakly reduced cccDNA. Lower doses of MAP30 inhibited the expression of HbsAg and HBeAg. A higher dose of MAP30 was effective in suppressing viral replication by altering the kinetics of replicative DNA intermediates. MAP30 inhibited the production of HBV dose dependently (p < 0.01) and time-dependently (p < 0.001) [6].
PAP from Pokeweed americana also inhibited HBV replication in vitro. A eukaryotic expression plasmid (pXF3H-PAP) encoding PAP was constructed PAP was constructed which inhibited the level of HBeAg, HBsAg and HBV DNA in a dose-dependent manner in the Hep G2.2.15 cell line. PAP reduced levels of HBV mRNA, recognized and depurinated HBV-mRNA, which included the 3.5kb pregenome mRNA and 2.4/2.1 kb pregenome mRNA. PAP also reduced HBV nucleocapsid associated DNA in a dose dependent manner. At the DNA level, transfection of 1.0 and 2.0 μg plasmid pXF3H-PAP into HepG2 cells reduced levels of HBV nucleocapside-associated DNA by 38.0% and 74.0% respectively and reduced the levels of HBsAg and HBeAg in a dose dependent manner. Plasmid pXF3H-PAP reduced the level of HBeAg by 72.7% and 99.3% respectively after transfection of 1.0 μg and 2.0 μg into HepG2 cells. The level of HBsAg in the media were reduced by 76.8% and 99.7% respectively after transfection of 1 μg and 2.0 μg plasmid pXF3H-PAP into HepG2 cells [7].
-
Herpes simplex virus is a large double stranded linear DNA encased with an icosahedral protein cage. HSV-1 is an enveloped DNA virus and is the causative agent of several types of primary and recurrent diseases including herpes labialis, gingivostomatitis, keratoconjunctivities and encephalitis. HSV-2 is the major cause of genital herpes; 78-97 percent of HSV-2 infections are asymptomatic. Genital herpes usually spreads through patients with asymptomatic or subclinical infections [17] MAP30 was effective against HSV-1, HSV-2 as well as an HSV specific nucleoside analog cyclovir (ACV)-resistant strain. In HIV patients, HSV infections are much more severe, frequent and life-threatening. In HIV patients, acyclovir (ACV, a HSV specific nucleoside analog) is used to treat HSV infections but they become resistant to ACV due to alterations in the viral DNA polymerase or mutation of the viral enzyme thymidine kinase [18]. HSV exposed fibroblasts started to display signs of cytolytic changes characterized by rounding and clumping of cells. In the presence of MAP30 cytopathic effects were inhibited and similar results were observed with ACV. MAP30 (EC50μm) demonstrated an exceptionally high activity against wild type HSV-2 strain, which is about 20-fold more potent than ACV. MAP30 were effective against ACV-resistant HSV at doses similar to those that were effective against wild type herpesviruses. MAP30 antiherpetic activity against ACV-resistant strains was two to three logs more potent than that of ACV [3].
Trichosanthin, a type Ⅰ ribosome inactivating protein from Trichosanthes kirilowii, was effective against HSV-1 replication. TCS blocked phospho rylation of p38MAPK and Bcl-2 in Vero cells which were induced by HSV-1. The suppression of p38 and Bcl-2 was the cause of reduction in cell viability and viral replication. The further addition of p38 MAPK inhibitor SB203580 produced reduction in Bcl-2, p38 MAPK, viral replication and Vero cell viability. HSV-1 infected Vero cells showed apoptosis when treated with TCS. The suppression of Bcl-2 by TCS and resulted in the signal to the cells changing from prosurvival to apoptosis. The reduction in viral replication was due to increased apoptosis in the HSV-1 infected cells [9].
-
PAP inhibits HTLV-I gene expression and has no cytotoxicity effects. Human T-cell leukemia virus I (HTLV-I) is a deltavirus that causes adult T-cell leukemia and neurological disorders like tropical spastic paraparesis. Also, HTLV-I-associated infected individuals have a 2-3% estimated lifetime risk of developing adult T-cell leukemia with a period of latency from 20-30 years. Leukemia progresses after clonal expansion of T-cells infected with the virus and the disease appear mainly in individuals who have been infected with the HTLV-I virus early in life [26]. There is no effective antiretroviral treatment to restrict the development of disease associated with HTLV-I.
PAP efficiently inhibited HTLV-I gene expression at both translational and transcriptional levels and exhibited no cytotoxicity towards the host cell. PAP reduced the synthesis of viral proteins in part by decreasing the translational efficiency of HTLV-I gag/pol mRNA by depurinating nucleotides within the gag open reading frame. Viral mRNA level was reduced due to the decreased amount of viral transactivator protein, Tax, leading to feed-back inhibition of HTLV-I gene expression by diminishing gag/pol, env and tax/rex mRNA levels. Therefore, PAP efficiently diminished virus production due to suppression of HTLV-I gene expression at both translational and transcriptional level [15].
-
Trichosanthin, purified from Trichosanthes kirilowii, was formulated for clinical use and used in a multicentre dose-escalation study in HIV-infected patients. Trichosanthin was used to treat 51 HIV-positive individuals and patients who received three doses of trichosanthin had significant increases in CD4+ T-cell levels and reduction in ESR levels. Furthermore, HIV p24 antigen level decreased in 16 out of 18 patients who entered the study with positive values. During the first two intravenous injections trichosanthin, reversible hypoalbuminaemia, fatigue and myalgias side effects were seen but become more severe after the third infusion. Alteration in mental status occurred as dementia in six out of 51 patients and progressed to coma in two of these. One patient entered a coma and died of aspiration pneumonia during the recovery phase. Most patients with these complications had abnormal MRI scans with findings indicative of central nervous system infarcts, which may reflect high level of HIV infection in the brain. It was presumed that non-specific neuronal damage or specific destruction of HIV-infected cells in the CNS possibly associated with weakened blood-brain barrier. These side-effects were likely not related to trichosanthin because one patient who self-administered 104 mg/kg of trichosanthin had no such side effects [4].
GLQ223 is a highly purified formuation of trichosanthin. GLQ223 was administered intravenously at doses of 8, 16, 24, 36 and 50 µg/kg of body weight in 22 HIV positive patients. CD4+ T-lymphocyte levels were increased after the first dose of GLQ223 and persisted for at least 4 weeks after the last infusion. The significantly increased CD4+ T-lymphocyte observed in patients treated with GLQ223 < 36µg/kg, however numerical increase was greater in those patients treated with ≥36 µg/kg. CD8+ T-lymphocyte level decreased in patients after the first infusion β2-microglobulin levels increased during the infusions and then declined when infusion was ended. Patients reported flu-like syndrome characterized by muscle and joint aches and an increase in creatinine kinase levels. Anti-GLQ223 antibody levels were detected in most patients and greatest increase in β2-microglo bulin and CD4+ T-cell levels were observed after the third and fourth doses of GLQ223. The majority of side-effects were classified as mild, reversed with simple treatments and repeated dosing with GLQ223 was safe [10].
TXU (anti-CD7)-PAP showed favorable pharmaco kinetics in HIV infected patients with a long plasma elimination half-life of 12.4±1.4h and slow systemic clearance of 2.7±0.7 mL/h/kg. Patients treated with a single 5µg/kg dose of TXU-PAP showed potent anti-HIV activity in vitro and inhibited HIV replication in normal peripheral blood mononuclear cells (PBMCs) even at a 1: 100 dilution. At this very low dose level TXU-PAP treatment increased circulating NK cell numbers and reduced viral burden [27].
Trichosanthin (TCS)
Pokeweed antiviral protein
Momordica antiviral protein (MAP30)
Hepatitis B virus (HBV)
Herpes simplex virus (HSV)
Human T-cell leukemia virus
phase Ⅰ/Ⅱ clinical trials using RIPs
-
Many ribosome inactivating proteins exhibit RNA N-glycosidase activity and depurinate large ribosomal RNA from ribosomes, thus arresting protein synthesis. RIPs have a broad range of antiviral activity against different viruses including human immunodeficiency virus, hepatitis B virus and herpes simplex virus. Research on RIPs has received particular attention in recent years because their biological mechanism against virus remains unknown and also for their effective use as immunotoxins for the target therapy of important disease. How RIPs effect anti-viral activity is still unclear. Most antiretroviral approaches have focused on HIV-specific targets such as reverse transcriptase, protease and gp120. In such a situation, it is important to investigate the potential of RIPs in therapeutic intervention as HIV is a complex virus and develops drug resistance at various stages in its life cycle.
The phase Ⅰ/Ⅱ trial demonstrates the potential of trichosanthin for treating HIV positive patients. The anti-HIV activity of trichosanthin revealed that HIV replication mechanisms are more sensitive than cellular mechanisms to ribosome inactivating proteins. The construction of a RIP conjugate (immunotoxin) may lead to improved biodistribution characteristics. The mechanism of action of TXU-PAP is different from other available anti-HIV agents; combination of TXU-PAP with anti-HIV drugs may enhance their anti-HIV activity.













 DownLoad:
DownLoad: