HTML
-
Since 2009, a newly emerging infectious disease has been brought into focus of public health in China (Yu et al., 2011). The clinical manifestations of this disease included fever, thrombocytopenia, and leukocytopenia, and mortality rates ranging from 6% to 30% were reported (Yu et al., 2011; Li et al., 2014; Park et al., 2014; Chang et al., 2013). Accordingly, this disease was designated as severe fever with thrombocytopenia syndrome (SFTS). Although its clinical manifestations resembled the human anaplasmosis (Zhang et al., 2008), neither DNA nor antibodies against human anaplasmosis could be detected in blood samples from the majority of the patients with SFTS. The pathogen responsible for this infectious disease was identified as a novel phlebovirus of Bunyaviridae family, which was initially given different names, including SFTS virus (SFTSV), Dabie Mountain virus (DBM virus), Henan fever virus, or Huaiyangshan virus (HYSV) (Yu et al., 2011; Stone, 2010; Zhang et al., 2012; Xu et al., 2011); the virus was finally named as SFTSV (Yu et al., 2011). Thereafter, similar viruses have also been reported in Japan, Korea, and the United States of America (USA) (Takahashi et al., 2014; Kim et al., 2013; Mcmullan et al., 2012). In addition, other newly emerged phleboviruses were isolated from patients in USA in 2012, including the Heartland virus and a new phlebovirus, which was genetically similar to SFTSV (Mcmullan et al., 2012).
Electron microscopy has shown that SFTSV particles are spherical and measured 80-100 nm in diameter (Yu et al., 2011). The virions possess a unit of the membrane envelope from which polypeptide spikes (5-10 nm long) protrude (Yu et al., 2011). Although the transmission hosts and vectors of SFTSV remain unclear, Haemaphysalis longicornis is considered the most likely transmission vector (Luo et al., 2015). Several studies have shown that SFTSV can undergo tachytely evolution via genetic mutation and recombination (Ding et al., 2013; He et al., 2012). Moreover, microbial evolution and social and environmental factors seriously affect these emerging and re-emerging infectious diseases. Thus, SFTS is still recognized as a major threat to public health.
-
The genome of SFTSV is a single-stranded negativesense RNA and comprises three segments (S, M, L). The S segment, which contains 1744 nucleotides of ambisense RNA, harbors two nonoverlapping open reading frames (ORFs) encoding nucleocapsid protein (NP) and nonstructural proteins (NSs), in opposite directions, separated by a 62-bp intergenic region. NP is involved in viral replication and transcription and can form intracellular inclusion bodies in infected THP-1 cells (Jin et al., 2016). The nucleoprotein encapsidates and packages genomic RNA into ribonucleoprotein complexes to protect the RNA from degradation by exogenous nucleases or immune system components in the host cell (Sun et al., 2012). NSs regulate host innate immune responses and suppress interferon (IFN)-β responses (Wu et al., 2014). Moreover, NSs interact with one another and with NP and are associated with viral RNA in infected cells, suggesting that NSs may be directly involved in viral replication (Wu et al., 2014). Additionally, some studies have shown that synaptogyrin-2 can promote the replication of SFTSV through interacting with viral NS protein (Sun et al., 2016). Both the nucleoprotein and the NS protein of SFTSV prevent the antiviral immune response in host cells by blocking the activation of IFN-β and nuclear factor κB signaling (Qu et al., 2012) (Table 1).
Segment bp ORF Position Direction Encoded protein Function L 6368 1 17-6271 5′-3′ RdRp Replication, transcription, and viral cap-dependent transcription. M 3378 2 19-1704 5′-3′ Gn Virus assembly, formation of virus particles, immunogenicity, neutralizing or protective epitopes, viral infectivity, precursor of glycoprotein. 1705-3240 5′-3′ Gc S 1744 2 29-910 5′-3′ NSs Host innate immune responses, formation of viral inclusion bodies, formation of viroplasm-like structures, virus replication, transcription, replication, virion assembly, formation of RNP, viral RNA encapsidation. 1702-965 3′ -5′ NP Note: *With references to: Liu et al., 2014; Lei et al., 2015; Li et al., 2011 Table 1. Genomic characteristics of SFTSV*
The M segment contains 3378 nucleotides, encoding a 1073-amino acid glycoprotein (Gn and Gs) precursor, which contains immunogenic, protective, or neutralizing epitopes (Wang et al., 2012). Plegge and colleagues provided evidence showing that processing of the SFTSV Gn/Gc polyprotein is critical for viral infectivity and requires an internal Gc signal peptide (Plegge et al., 2016). A neutralizing monoclonal antibody (anti-SFTSV) was found to bind a linear epitope in the ectodomain of glycoprotein Gn of SFTSV. Its role is known to block the interaction between the Gn protein and the cellular receptor (Guo et al., 2013) (Table 1).
The L segment, which contains 6368 nucleotides, has one ORF encoding an RNA-dependent RNA polymerase (RdRp) with 2084 amino acids. RdRp facilitates the replication and transcription of the viral RNA. Phylogenetic analysis of SFTSV sequences indicated that this virus represents a new independent branch within Phlebovirus, roughly equidistant from the already established sandfly fever and Uukuniemi viruses (Richard et al., 2014) (Table 1).
Zhang and Xu (2016) reported that the status of SFTSV infection from domestic and wild animals was related to transmission from different tick species. They proposed a tick and migratory bird model for SFTSV transmission and a hypothesis for the local transmission and wide dissemination of SFTSV by combining infection data with host and vector ecology data. They also showed that the risk of infection is reduced if the animals and vector populations are monitored (Zhang and Xu, 2016).
The distribution of H. longicornis ticks and the migratory routes of four wild fowl across China, South Korea, and Japan are coincident. Therefore, a tick and migratory bird model for the transmission of the SFTSV was advocated (Guan et al., 2016; Zhang and Xu, 2016). Zhang et al. (2012) sampled 613 adult ticks, including 88.09% H. longicornis and 11.91% Boophilus microplus, which were collected from domestic animals around the villages in four counties (Dawu, Zengdu, Xinzhou, and Xinxian) of Hubei and Henan Provinces in central China, where confirmed patients lived in the nearby districts. Among these samples, the positive ratios of SFTSV were 4.93% in H. longicornis and 0.613% in R. microplus tick pools (Zhang et al., 2012). However, H. longicornis ticks with SFTSV-positive were only discovered in SFTSV endemic places, whereas SFTSV-positive R. microplus ticks were detected in both endemic and non-endemic regions from some counties in Henan and Hubei Provinces. In South Korea, H. longicornis (90.8%) ticks were dominant, followed by H. flava (8.8%), Ixodes nipponensis (0.3%), and I. persulcatus (0.05%) (Park et al., 2014). The positive ticks were distributed in the country, and an SFTSV-infection case was confirmed in Gangwon Province, South Korea, where positive ticks had been previously discovered (Kim et al., 2013; Park et al., 2014; Zhang et al., 2016). Based on these surveys, H. longicornis was speculated to be the major vector of SFTSV. However, SFTSVpositive arthropods may be additional vectors of SFTSV or may obtain the virus from the blood of SFTSV-infected animals (Niu et al., 2013). Therefore, further studies of arthropods are needed to determine whether they act as vectors of SFTSV.
Animal hosts of SFTSV are still unclear. SFTSV has a wide host range and infects several domesticated animals in endemic areas. In a seroepidemiological survey of domestic animals in Jiangsu Province, researchers collected samples from goats (57%), cattle (32%), dogs (6%), pigs (5%), and chickens (1%) (Liu et al., 2014) and found that the seroprevalence of SFTSV was 69.5% for sheep, 60.5% for cattle, 37.9% for dogs, 3.1% for pigs, and 47.4% for chickens in Shandong Province (Niu et al., 2013). Moreover, rodents have seropositive for SFTSV in Jiangsu Province, with 6.9% seroprevalence in wild rodents and 7.87% in house rodents (Ge et al., 2012), including house shrews (Liu et al., 2013), mouse (Mus musculus), rat (Rattus norvegicus) (Yun et al., 2015; Ge et al., 2012), and field mouse (Apodemus agrarius) (Ni et al., 2015). In the USA, a seroepidemiological survey showed that the positive rates of anti-Heartland virus antibodies in samples from cattle, sheep, goats, deer, and elk were 16%, 13%, 11%, 12%, and 18%, respectively (Li et al., 2014). SFTSV viral RNAs were also tested in animal species, but typically at very low levels, and ranged from 1.7% to 5.3% (Niu et al., 2011). Therefore, both domestic and captive farmed animals in some areas may be exposed to SFTSV, or Heartland virus (Mcmullan et al., 2012). Many wild animals, such as deer, weasels, hedgehogs, brushtail possums, and some bird species, are regular hosts of ticks (Yun et al., 2015).
Although there is no evidence that the virus can cause disease in animals, blood from subclinically infected animals could be a source of infection (Mishra et al., 2011). Bao and colleagues reported a family in which SFTSV underwent person-to-person transmission through the blood (Bao et al., 2011). Subsequently, a cluster of humanto-human transmission cases of SFTSV was reported in Shandong, Jiangsu, and Anhui Provinces in China; this transmission may have been related to direct contact with the blood of patients or aerosol through the mucosa (Jiang et al., 2015; Gong et al., 2015). However, the mechanisms of this person-to-person transmission event are still unclear. Therefore, veterinarians and slaughterhouse workers may be at high risk of SFTSV infection.
-
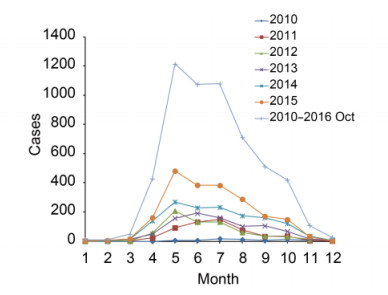
SFTS infectious disease was first reported in rural regions in Hubei and Henan Province of central China in March and July of 2009 (Zhang et al., 2013). However, the first case of SFTS in Dingyuan County, Anhui Province was reported in September of 2006 (Liu et al., 2012). From 2010 to October 2016, SFTS cases were reported in 23 provinces and experimentally confirmed in 18 provinces by the Chinese Disease Prevention and Control Information System (Figure 1). The highest numbers of reported cases occurred in Henan (37%), Shandong (26.6%), Anhui (14%), and Hubei (12.6%), and 97.7% of SFTS cases were distributed mainly in individuals of age 25-80 years. Outbreaks of SFTS occurred mostly from March to November, and the main epidemic period was from May to July, with a peak in May (Figure 2).
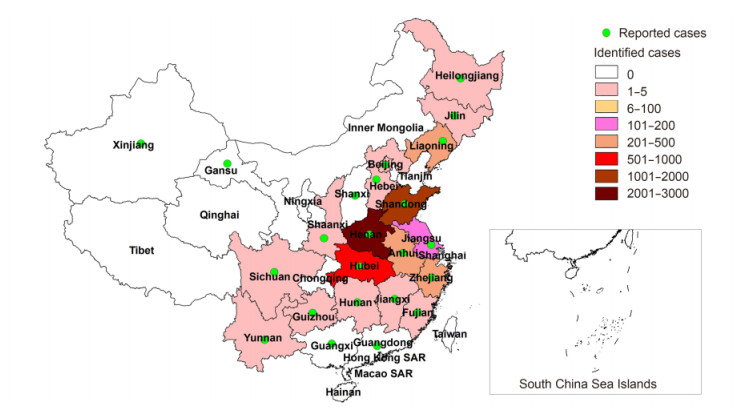
Figure 1. SFTS reported and confirmed cases in China (Data from the Chinese Disease Prevention and Control Information System, 2010 to October 2016)
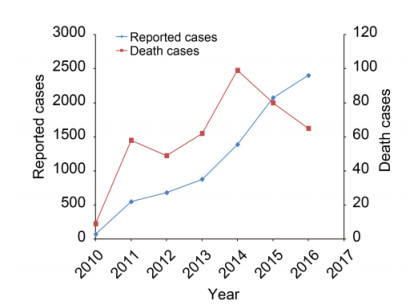
From 2013 to October 2016, SFTS cases in China significantly increased gradually; 7419 cases occurred in China, with 355 deaths, mainly distributed in eastern and central China. An annual report of SFTS cases from Anhui, Henan, Hubei, and Shandong Provinces showed that there were over 1000 cases since 2014. The national average mortality rate of SFTS infection in China was 5.3% from 2010 to October 2016 (Figure 3).
The female-to-male ratio of SFTSV infection is 1.16:1. The ages of patients with SFTS range from 5 to 87 years (median 61 years) (Liu et al., 2014; Ma et al., 2015), and the annual incidence increased with age (Ma et al., 2015). Notably, farmers in endemic areas are at a higher risk (87.6%), and almost 97% of patients with SFTS are farmers residing in wooded and hilly areas (Liu et al., 2014; Ma et al., 2015). Among patients, it was reported that 21.85% recalled for a tick bite within two weeks; the other patients did not memorize (Xu et al., 2011). The latent period is generally 7-14 days (average of 9 days) (Gai et al., 2012). An epidemiological survey showed that about 1-3% of the population is infected with SFTSV in popular areas. SFTSV mainly infects older individuals, and death is most frequent in patients older than 50 years (79%), suggesting that SFTSV infects people with low immunity. Indeed, age may be a key risk factor for death and hospitalization in patients with SFTS (Lei et al., 2015).
SFTSV has broad genetic diversity. Because of the absence of a proofreading function in the viral RNA replication and transcription processes, SFTSV shows high mutation rates during replication (Lam et al., 2013), which may provide a basis for its genetic diversity (Zhang et al., 2011). Interestingly, the evolutionary rates are different from three segments of SFTSV. The highest and lowest evolutionary rates are found in the S and L segments, respectively (Liu et al., 2016). Using the average evolutionary rate, researchers showed that SFTSV was present 20-87 years ago in the Dabie Mountains, China and spread to some other places of China, Japan, and South Korea (Liu et al., 2016). Moreover, the virus underwent frequent selection through constant reassortment (Liu et al., 2016). Phylogenetic analysis of SFTSV strains isolated from 2009 to 2016 suggested that SFTSV strains could be divided into six sublineages (A-F; Figure 4A-C). The strains from South Korea were divided into four sublineages (A, B, D, and F), and the Japanese strains were divided in three sublineages (C, D, and F). The strains belonging to lineage A came from China, including Hubei, Henan, Jiangsu, Shandong and Liaoning Provinces except for three strains (KASJH/South Korea/2014, Ganwon/South Korea/2012, and KR 1020150042419-A/2). We also identified segment reassortment as the same strains belonged to different lineages. The AHL strain ascribed to lineage C in segments M and L, but to lineage B in segment S. Similarly, the S and L segments of the LN2012-14 strain ascribed to the B lineage, whereas the M segment of LN2012-14 ascribed to the A lineage. Phylogenetic trees manifested that the Zhejiang/ 01/2011 strain was closely related to strains from South Korea and Japan. These researches showed that human behavior pattern and various activities may provide an important role for SFTSV cyclic transmission among China, Japan, and South Korea.
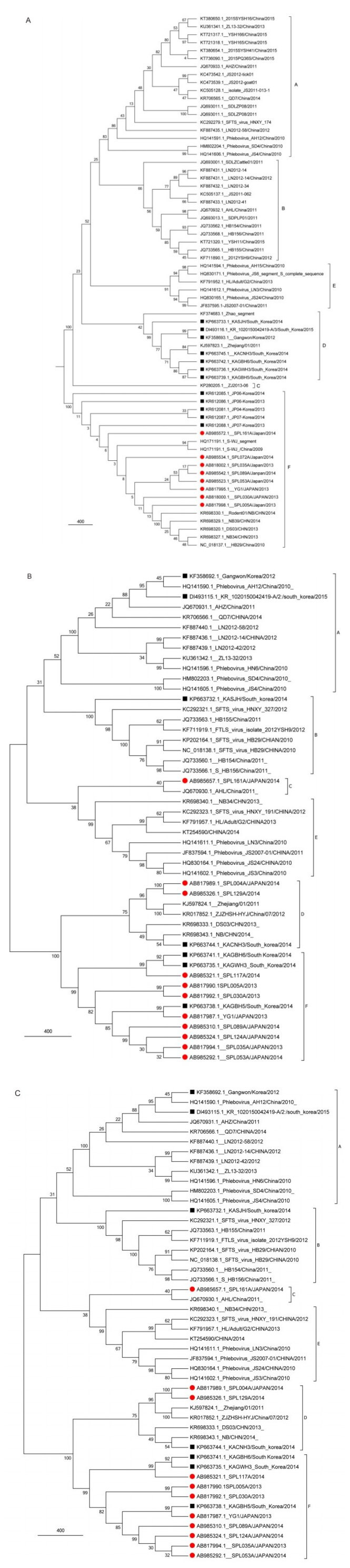
Figure 4. Phylogenetic analysis of the whole S (A), M (B), and L (C) segment sequences of SFTSV strains. The maximum likelihood trees were constructed using MEGA 6.06. GenBank accession numbers and strains are labeled on each branch. SFTSV was classified into six lineages (A-F) according to the genome segments. Red represents the isolates from Japan, and the black framing represents isolates from South Korea.
A sublineage of lineage 1 has been shown to contain a unique M segment recombined from two of three SFTSV lineages (He et al., 2012). Ding et al. discovered that the S segment with two SFTSV strains had a different recombined origin from L and M (Ding et al., 2013). Many studies also found that the viruses could experience rapid evolution via gene mutations and recombination (He et al., 2012). Moreover, although homologous recombination is known to be rare in negative-strand RNA viruses, intragenic recombination may have an important role in the rapid evolution. In segmented-genome viruses, reassortment may be an efficient process, resulting in critical adaptions, recombinations, and mutations in a single step. This could also allow major changes in adaptive space, leading to alterations in host tropism.
Reassortment of genome segments has been observed within vertebrate animal, plant, and arthropod hosts in the Bunyaviridae family (Webster et al., 2011). However, it is unclear whether reassortment can affect the rapid evolution of SFTSV. These studies may improve our knowledge and understanding of the molecular mechanisms of viral genetic diversity and interspecies transmission.
-
The clinical manifestation of SFTS is nonspecific and difficult to distinguish from those of human granulocytic anaplasmosis (HGA), leptospirosis, and hemorrhagic fever with renal syndrome (HFRS) (Zhang and Xu, 2016). The major clinical symptoms of SFTS include fever, gastrointestinal symptoms, myalgia, hemorrhage in the mouth, and regional lymphadenopathy. In addition, proteinuria (84%), hematuria (59%), dizziness (23.52%), headache (19.2%), and chills (10.29%) are also common symptoms in SFTSV-infected patients (Yu et al., 2011). Moreover, transient myocardial dysfunction and encephalopathy can occur beyond the major clinical symptoms of SFTS (Takeshi et al., 2016; Cui et al., 2015). The main clinical manifestations on laboratory detection include thrombocytopenia (95%) and leukocytopenia (86%). We also listed our data for laboratoryconfirmed clinical symptoms in SFTSV-infected patients (Table 2). In general, the decreased population and dysfunction of monocytes in patients with acute-phase SFTS patients may lead to more severe disease (Cheng et al., 2016). Liu and colleagues found clinical indicators that were associated with seriously outcomes; however, they found no proof to indicate the use of ribavirin in the treatment for SFTSV infection (Liu et al., 2013).
Symptoms Patients with symptoms (N=68)
No. (%)Fever 68 (100.00) Malaise 49 (72.06) Fatigue 48 (70.59) Anorexia 39 (57.35) Nausea 21 (30.88) Diarrhea 19 (27.94) Vomiting 17 (25.00) Dizziness 16 (23.53) Headache 13 (19.12) Throat congestion 13 (19.12) Cough 12 (17.65) Myalgia 8 (11.76) Chill 7 (10.29) Lymphadenopathy 7 (10.29) Table 2. Clinical symptoms of patients with laboratory confirmed disease in Hubei Province, 2011
SFTSV strains have been isolated using DH82, Vero, or Vero E6 cells. After infection with SFTSV, the DH82 cells attached to the culture flask. However, early detection of SFTSV infection is crucial for improving the survival rates and prevention of viral transmission. Early detection is based on the epidemiological characteristics of the disease, such as epidemic season, geographical location, clinical symptoms, history of tick bite, and laboratory examination. If the clinical manifestations of SFTSV infection are nonspecific, laboratory confirmation is essential.
A lot of methods were used to amplify viral RNA, including reverse transcription polymerase chain reaction (RT-PCR) and RT-loop-mediated isothermal amplification (RT-LAMP) (Zhang et al., 2011; Webster et al., 2011; Yun et al., 2014). Amplification of the segment S is the most sensitive RT-PCR assay (Chi et al., 2012; Cui et al., 2012; Sun et al., 2012; Yang et al., 2012). The RT-PCR primers for the viral S segment are more sensitive than those for the L segment, which may be related to the lengths of the primers (Wen et al., 2014). Quantitative real-time PCR (qRT-PCR) for SFTSV infection has a lower contamination rate and higher sensitivity and specificity. Moreover, this method is also quicker than conventional one-step RT-PCR, and the identification of the SFTSV genome in the blood using qRT-PCR during the acute phase of SFTSV infection may be used as an indica tor to forecast the outcomes of SFTS (Yoshikawa et al., 2016; Li et al., 2013). Li and colleagues (2016) reported that rapid diagnosis of SFTSV by AllGlo probe-based real-time RT-PCR showed high sensitivity, specificity, and stability. SFTSV was detected by RT-nested PCR to amplify the S or L segment of the SFTS viral RNA gene (Wen et al., 2011). Furthermore, a multiplex real-time RT-PCR assay could simultaneously detect four viral hemorrhagic fever pathogens (SFTSV, Hantan virus, Seoul virus, and dengue virus) (Li et al., 2013). A simple technique that incorporated RT-cross-priming amplification (RT-CPA) connected to a vertical-flow visualization strip was used to rapidly detect SFTSV infection (Chi et al., 2012) (Table 3).
Category Detection methods Minimum detection limit Sensitivity Specificity SFTSV S segment qRT-PCR (TaqMan) 10 copies/μL 98.6-100% 99-100% SFTSV L segment qRT-PCR (TaqMan) 5 × 10 copies/μL NA NA SFTSV M segment qRT-PCR (TaqMan) 2 × 102 copies/mL NA NA SFTSV M segment RT-LAMP 10 copies/test NA 100% SFTSV M segment RT-CPA-VF 100 copies/test 94.1% 100% SFTSV S segment qRT-PCR (AllGlo) 1 × 103 copies/mL NA NA SFTSV S & L segment Nest RT-PCR 10 copies/μL NA 55% SFTSV antibody & RNA ELISA+Nest RT-PCR NA NA 86% SFTSV antibody IFA NA 95.6% 74.29% SFTSV IgG MLBI NA 90.7% 66.67-100% SFTSV total antibody ELISA NA 100% 99.57-100% SFTSV RNA Luminex 102 copies/μL NA NA SFTSV IgM ELISA NA 90.59-96.67% 59-100% SFTSV IgM ICA NA 98.4-100% NA SFTSV antigen ELISA 0.117 mg NA NA SFTSV RNA qRT-PCR (MGB) 10 copies/μL 100% 99% SFTSV IgG ICA NA 96.7-98.6% NA Note: NA, not applicable; ICA, immunochromatographic assay; RT-LAMP, reverse transcription-loop-mediated isothermal amplification; RT-CPA, reverse transcription-cross-priming amplification; MLBI, multiplexed luminex-based immunoassay; VF, vertical flow; MGB, minor-groove-binding (Yu et al., 2015; Wang et al., 2014; Cui et al., 2012; Wu et al., 2014; Li et al., 2013; Wen et al., 2011). Table 3. Comparison of various detection methods for SFTSV
Specific antibodies to SFTSV are detectable from about 1 week after disease onset, and SFTSV-specific IgG antibodies can surge to peak levels by 6 months, remaining detectable for at least 5 years; however, SFTSVspecific IgM antibodies can surge to peak levels by 4 weeks and last for about 1 year after infection (Li, 2011; Lu et al., 2015). Patients with SFTS show apparent loss of T, B, and natural killer (NK) cells during the first week of infection, which are rapidly recovered to standard levels. A markedly declined level of humoral immunity was observed simultaneously in patients with severe SFTS, particularly during the acute phase of the SFTSV infection (Lu et al., 2015). SFTSV-specific IgG, IgM and total antibodies have been detected by in-house Mac-enzyme-linked immunosorbent assays (ELISAs), indirect ELISA assays, double antigen sandwich ELISAs, and immunofluorescence assays (IFAs) (Li et al., 2013; Lei et al., 2015; Jiao et al., 2011). These methods have higher sensitivity than neutralization tests, and no crossreaction among SFTSV, hantavirus, and dengue virus was observed (Jiao et al., 2011). Immunochromatographic assays (ICAs) may be suitable measure for rapid, on-site, and inexpensive detection SFTSV infection, and commercially available kits show good specificity for the diagnosis of SFTSV-specific IgM and IgG with no crossreaction with positive serum samples in patients with infections of Japanese encephalitis virus, Dengue virus, Hantavirus, hepatitis C virus (HCV), human immunodeficiency virus (HIV), hepatitis B virus (HBV), or Mycobacterium tuberculosis (Wang et al., 2014). Serum neutralization tests of SFTSV have been reported, e.g., 50% plaque reduction neutralization tests (PRNT50) and microtiter neutralization tests. Huang and colleagues (Huang et al., 2016) identified that hospitalized patients with SFTSV infection maintained neutralizing antibodies to SFTSV for 4 years after diagnosis.
-
SFTS is a severe disease found in many countries worldwide, including China, Japan, and South Korea, with central China as the main epidemic region. However, SFTSV is undergoing rapid evolution owing to gene mutation, reassortment, and recombination in tick vectors and animal hosts. The wide epidemic, rapid evolution, cross-species transmission, and the higher case fatality rates have made SFTS a significant public health problem. The role of climatic factors, cross-species transmission, and the development of vaccines and new drugs may be hotspots of future research.
-
This study was supported by grants from the China Mega-Project for Infectious Diseases (2012ZX10004-207), the China-US Collaborative Program on Emerging and Re-emerging Infectious Diseases (S2012Y03), the key project of the Health Ministry of Hubei Province (JX5A06), and the Hubei Provincial Outstanding Medical Academic Leader program.
-
The authors declare that they have no conflicts of interest. The study was approved by the Ethics Committees of the Centers for Disease Control and Prevention of Hubei Province. Written consents were obtained from all participants involved in the study.














 DownLoad:
DownLoad: