HTML
-
Acquired immune deficiency syndrome (AIDS), caused by infection with the human immunodeficiency virus (HIV) (Sepkowitz 2001), has become a major global health concern and social problem due to its associated high mortality and rapid viral transmission (Fauci 2008; Fettig et al. 2014; Maartens et al. 2014; Saag and Masur 2014). Since the first case reported in China (Wu et al. 2004), there has been a tendency toward an increase in HIV/AIDS epidemics in the country (Wang 2007; Wang et al. 2008; Zhang et al. 2013), and currently, approximately 810, 000 individuals are estimated to have HIV/AIDS (NCAIDS and NCSTD 2017).
Since 2010, HIV/AIDS has been further prioritized by the government of China, and the coverage for HIV/AIDS detection has been extended with the aims of identifying HIV infections and AIDS patients and reducing secondary HIV transmission (Yan et al. 2016). Due to an increase in detection and the multiple origins of samples, there has been an increase in the number of patients with indeterminate Western blot (WB) results (Han et al. 2016; Liu et al. 2016; Peng et al. 2014; Wei 2015). Data from the national detection of HIV antibodies reveal that the proportion of indeterminate WB results increased from 4.7% in 2007 to 7.9% in 2013 (Gao et al. 2016). According to the 2015 revised National Guidelines for Detection of HIV/AIDS, periodical detection of HIV antibodies should be implemented for subjects with indeterminate WB results (MOH 2016). Previous follow-up studies have highlighted the difficulty in follow-up and the low rate of re-examination in patients with indeterminate WB results (Carneiro-Proietti et al. 1999; Jackson et al. 1995; Jamjoom et al. 1997). This is not only problematic with respect to laboratory HIV testing and epidemic management but also markedly affects the effectiveness of posttest counseling. Moreover, it entails an unnecessary waste of resources, places a heavy psychological burden on patients, and delays the treatment.
Given that WB assays produce a large number of indeterminate results, several novel assays have been developed for detecting HIV infection, including the INNO-LIA HIV Score assay (Tuaillon et al. 2017), the recombinant immune blot assay (RIBA) (Kline et al. 1996), and qualitative or quantitative HIV nucleic acid testing (Coste et al. 1996; Yamazaki et al. 2016; van Gemen et al. 1993). However, the effectiveness of these assays for the differential diagnosis of HIV-indeterminate WB samples has hither to been undetermined.
The present study was therefore designed with the aim of investigating the proportion of indeterminate WB results for antibodies against HIV in Fujian Province, Southeastern China, and evaluating the effectiveness of quantitative and qualitative nucleic acid tests on HIV indeterminate WB samples.
-
During the period from 2015 through 2016, a total of 6360 positive HIV screening samples were collected from HIV-suspected individuals in Fujian Province, who were screened by local hospitals, clinics, voluntary counseling and testing (VCT), and blood centers. These samples were subsequently subjected to confirmatory HIV testing. All confirmatory HIV testing data in Fujian were obtained from the National HIV/AIDS Information System in China (Mao et al. 2010). The demographic characteristics, history of contact, health, and antiretroviral therapy (ART) status of individuals were determined based on epidemiological surveys.
-
The anti-HIV antibody detection strategy and procedure of the confirmatory test and evaluation of the results were strictly in accordance with the 2015 revised National Guideline for Detection of HIV/AIDS (MOH 2016). All positive HIV screening samples were subjected to confirmatory HIV testing using an HIV Blot 2.2 WB kit (MP Biomedicals Asia Pacific Limited, Singapore). A positive result in HIV-1 antibody test was defined in terms of the detection of Env (gp160/gp41 and gp120) and Gag (p17, p24, p55), or Env (gp160/gp41 and gp120) and Pol (p31, p51, p66) genes. An HIV-indeterminate WB result was defined as any viral-specific bands present for which the banding pattern failed to meet the criteria for positivity, whereas a negative HIV-1 antibody test was defined by an absence of viral-specific bands.
-
The HIV-indeterminate WB samples were re-examined once each month after the first diagnosis, until a WB confirmatory test revealed a positive or negative result. If indeterminate results were detected throughout the 3-month follow-up period, the follow-up was extended to 6 months. Positive conversion was defined as the transition of an indeterminate result to a positive detection of HIV antibodies, whereas negative conversion was defined as conversion from an indeterminate result to a negative detection for HIV antibodies.
-
Quantitative HIV nucleic acid analysis was performed using a Quantitative HIV-1 Nucleic Acid Test Kit (PCR fluorescence probe assay; Da An Gene Co. Ltd. of Sun Yatsen University, Zhongshan, China) following the manufacturer's instructions, for which an HIV RNA viral load of < 250 IU/mL was identified as a negative result and loads of 250 IU/mL and greater were considered positive.
A qualitative HIV nucleic acid test was performed for segmental amplification of three specific genes (gag, env, and pol) using a previously described reaction system and conditions (Wei et al. 2003). The presence of at least two specific bands was defined as a positive result, whereas the absence of any specific band was identified as a negative result, and the presence of only one specific band indicated an indeterminate result.
HIV-1 p24 antigen was detected in blood samples using an HIV-1 p24 ELISA Kit (Beijing Key-Bio Biotech Co. Ltd., Beijing, China) following the manufacturer's instructions. An HIV-1 p24 antigen level of 1.25 U/mL or greater was defined as a positive HIV test, whereas levels less than 1.25 U/mL were identified as negative.
The HIV antibody confirmatory test results obtained in the final follow-up were used as the reference standard to evaluate the effectiveness of p24 antigen detection and quantitative and qualitative HIV nucleic acid testing for the differential diagnosis of HIV-indeterminate WB samples.
-
Statistical analyses were performed using SPSS version 19.0 statistical software (SPSS Inc., Chicago, IL, USA). Differences in proportions were assessed for statistical significance using a Chi square test. Differences were considered statistically significant at a P value < 0.05.
Data Collection
Confirmatory HIV Testing
Follow-up
Differential Diagnosis of HIV-Indeterminate WB Samples
Statistical Analysis
-
Two hundred and ten subjects with HIV-indeterminate WB results were detected from 6360 positive samples obtained via HIV screening received confirmatory HIV testing in Fujian Province between 2015 and 2016. The proportion of HIV-indeterminate WB results was 3.3% (210/6360). There was a significant difference in the proportion of HIV-indeterminate WB tests among different populations (χ2 = 122.098, P < 0.001) (Fig. 1), with the highest being recorded for pregnant and lying-in women receiving physical examinations (16.67%, 22/132), followed by that for voluntary blood donors (8.82%, 30/340).
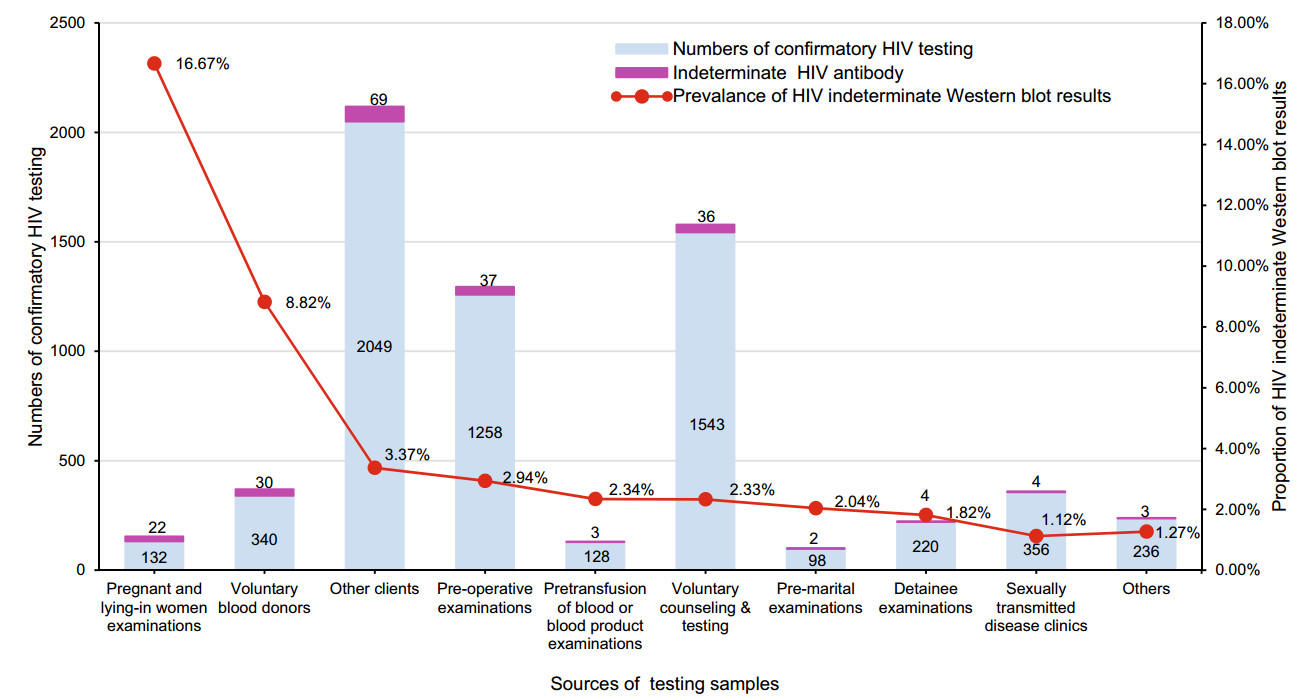
Figure 1. Detection of HIV-indeterminate Western blot results among different subject populations. Others include the spouse or sexual partner of HIV-positive patients, the children HIV-positive women, those with professional exposure, entertainment workers, paid blood donors, entry-exit personnel, recruits and those enrolled in ad hoc surveys.
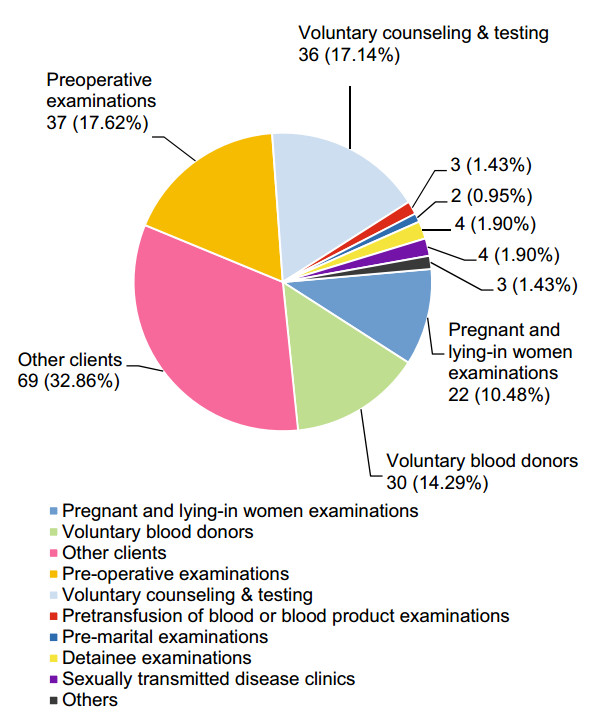
The HIV-indeterminate WB samples were mainly derived from other clients (32.86%), preoperative examinations (17.62%), and VCT (17.14%) (Fig. 2). The 210 subjects with HIV-indeterminate WB results comprised 142 men (67.62%) and 68 women (32.38%) who had a mean age of 39.95 ± 17.70 years. Of these 210 individuals, 112 (53.33%) reported no high-risk sexual behaviors, one (0.48%) was a child delivered by an HIV-infected woman, and 97 (46.19%) reported high-risk sexual behaviors (64.95% heterosexual behavior and 35.05% homosexual behavior). All subjects stated that they had not received ART during the first blood sampling after enrollment.
-
A total of 16 WB band patterns were detected among the 210 HIV-indeterminate WB samples, the three most common patterns of which were a single p24 band (44.29%), a doublet of gp160 and p24 (17.62%) bands, and a single gp160 band (14.29%) (Table 1).The Env protein showed 10 banding patterns in 98 HIV-indeterminate WB samples (46.67%), the Gag protein showed three patterns in 107 samples (50.95%), and the Pol protein showed three patterns in five samples.
Type of HIV protein Western blot band pattern No. subjects No. subjects receiving follow-up Follow-up outcomes No. of negative HIV antibodies No. of positive HIV antibodies No. of indeterminate HIV antibodies Env gp160 30 26 19 6 1 gp160 + p24 37 29 2 25 2 gp160 + gp120 9 7 2 4 1 gp160 + gp120 + gp41 15 6 0 4 2 gp160 + p24 + p17 2 2 0 2 0 gp160 + p55 + p51 1 1 1 0 0 gp160 + p66 + p51 + p24 1 1 0 1 0 gp120 1 0 0 0 0 gp41 1 1 1 0 0 gp41 + p24 1 1 1 0 0 Total 98 74 26 42 6 Gag p24 93 71 57 11 3 p24 + p17 4 3 3 0 0 p17 10 6 5 0 1 Total 107 80 65 11 4 Pol p51 3 1 0 1 0 p66 1 1 1 0 0 p66 + p51 1 1 1 0 0 Total 5 3 2 1 0 Total 210 157 93 54 10 Table 1. Western blot band patterns and follow-up outcomes in patients with HIV-indeterminate Western blot results.
There were 157 subjects with HIV-indeterminate WB results receiving follow-up until June 2017, which represented a follow-up rate of 74.76%, and the positive and negative conversion rates were 34.39% (54/157) and 59.24% (93/157), respectively. In addition, there were 10 samples (6.37%) for which indeterminate results were still obtained after a 6-month follow-up (Table 1).
Among samples with the common WB band patterns, the highest negative conversion rate of HIV antibodies (80.28%, 57/71) was seen in samples with a single p24 band, followed by that in samples with a single gp160 band (73.08%, 19/26). In contrast, the highest positive conversion rate (86.21%, 25/29) was found in samples with the doublet of gp160 and p24 bands. In addition, the highest negative conversion rate of HIV antibodies (81.25%, 65/80) was detected in samples with a Gag band, whereas the highest positive conversion rate (56.76%, 42/74) was seen in samples with an Env band (Table 1).
When we examined the seroconversion outcomes of HIV antibodies in patients with a different number of WB bands, we detected positive conversion rates of 16.98% (18/106), 70.73% (29/41), and 70% (7/10) in subjects with a single WB band, two bands, and three and more bands, respectively (Table 2). Trend analysis using a Chi square test revealed an increase in the possibility of a positive HIV antibody conversion with an increase in the number of WB bands (χ2 = 45.71, P < 0.001).
Western blot band pattern No. subjects No. subjects receiving follow-up Follow-up outcomes No. of negative HIV antibodies (%) No. of positive HIV antibodies (%) No. of indeterminate HIV antibodies Single band 139 106 83 (78.30%) 18 (16.98%) 5 Double bands 52 41 9 (21.95%) 29 (70.73%) 3 Three bands or more 19 10 1 (10%) 7 (70%) 2 Total 210 157 93 54 10 Table 2. Follow-up outcomes for HIV-indeterminate western blot samples with different numbers of Western blot bands.
We subsequently examined the seroconversion outcomes of HIV antibodies in different categories of patients with HIV-indeterminate WB results. We accordingly found that the negative conversion rate was 100% (19/19), 86.86% (20/23), and 73.08 (19/26) in pregnant and lying-in women receiving physical examinations, voluntary blood donors, and preoperative examinees, whereas the positive conversion rate of HIV antibodies was 53.33% (16/30) and 58.54% (24/41) in VCT and other clients, respectively. Furthermore, we detected a significant difference in the seroconversion outcomes of HIV antibodies among the different categories of patients with HIV-indeterminate WB results (χ2 = 30.212, P < 0.001).
-
A total of 157 individuals with HIV-indeterminate WB samples were successfully followed up, including sample from 54 individuals with positive conversion of HIV antibodies, 93 samples with negative conversion, and 10 samples with indeterminate WB results. Among the different assays assessed, quantitative HIV nucleic acid testing showed the highest sensitivity (96.3%, 52/54), followed by qualitative HIV nucleic acid testing (94.44%, 51/54). The lowest sensitivity (42.59%, 23/54) was obtained with p24 antigen detection. In terms of specificity, qualitative HIV nucleic acid testing was found to be the most specific (97.85%, 91/93), followed by p24 antigen detection (96.77%, 90/93). Lowest specificity (90.32%, 84/93) was obtained using quantitative HIV nucleic acid testing. The highest coincidence rate was obtained with qualitative HIV nucleic acid testing (96.6%, 142/147), followed by quantitative test (92.52%, 136/147), and was lowest (76.87%, 113/147) with p24 antigen detection (Table 3).
Western blot P24 antigen detection Quantitative HIV nucleic acid test Qualitative HIV nucleic acid test Total + - + - + - + 23 (42.59%) 31 (57.41%) 52 (96.3%) 2 (3.7%) 51 (94.44%) 3 (5.56%) 54 - 3 (3.23%) 90 (96.77%) 9 (9.68%) 84 (90.32%) 2 (2.15%) 91 (97.85%) 93 Total 26 121 61 86 53 94 147 Table 3. Performance of different assays for the differential diagnosis of HIV-indeterminate Western blot samples.
Proportion of HIV-Indeterminate WB Results
WB Band Patterns in HIV-Indeterminate WB Samples and Follow-up Outcomes
Effectiveness of Different Assays for Differential Diagnosis of HIV-Indeterminate WB Samples
-
HIV-indeterminate WB results are typically obtained when performing WB confirmatory assays, and there will be an increasing number of indeterminate samples with an increase in the rate of detection, which will present considerable challenges for the management of HIV/AIDS (Syed et al. 2005). The present study was therefore designed with the aim of investigating the epidemiology of HIV-indeterminate WB results in Fujian Province, southeastern China from 2015 to 2016. We found that the prevalence of HIV-indeterminate WB results in Fujian Province was 3.3%, which is lower than the proportion determined in the national detection of HIV antibodies (Gao et al. 2016).
Among 210 HIV-indeterminate WB subjects, the highest proportion was observed in pregnant and lying-in women receiving physical examinations (16.67%), followed by that in voluntary blood donors (8.82%), which is consistent with the findings of previous studies (Chu et al. 2013; Cremonezi et al. 2005; Granade et al. 2005; Yang et al. 2010; Zhang et al. 2015). In addition, the major WB banding patterns we observed in HIV-indeterminate WB samples were a single p24 band, gp160 and p24 double bands, and a single gp160 band. These three patterns were accounted for 76.19% of all indeterminate samples. The result was similar to those previously reported in China (Dou et al. 2015; Guan et al. 2016; Han et al. 2016; Miu et al. 2016; Zhu et al. 2015).
The prospective follow-up study revealed that the various origins of samples, WB band patterns, and the types of bands are indicative of the variation in the likelihood of HIV infection. In HIV-indeterminate WB samples obtained from pregnant and lying-in women receiving physical examinations or from voluntary blood donors, there was only a single gag (p24 or p17) or gp160 band in the WB, indicating a low rate of HIV infection. It is assumed that HIV-indeterminate results are mainly caused by nonspecific reactions, which are generally believed to be attributable to the particular status of individuals, such as that associated with specific physiological conditions, pathogenic microbial infections, and immunological or genetic factors. If subjects with indeterminate results have no high-risk sexual behaviors, they are apt to receive negative detection counseling, and follow-up re-examinations are required to exclude HIV infection following the standard guidelines. Nevertheless, if the detection samples are derived from VCT and other clients, and the WB banding pattern includes an Env band or there are two or more bands such as gp160 and p24, there is a high possibility of HIV infection. This may be associated with the early or late stage of HIV infection. It is generally considered that not all HIV-specific bands are present during the early stage of infection, and that if AIDS progresses to the end stage, the host immune system is substantially damaged, and the HIV antibody titer is reduced, resulting in the weak reactions of several specific bands or no reactions. Therefore, those subjects with HIV-indeterminate WB results should undergo epidemiological surveys. If the subjects have high-risk sexual behaviors or AIDSassociated clinical symptoms, they are inclined to receive positive test counseling, and the follow-up should be strengthened. The follow-up period should be shortened to 1 to 2 weeks, and a second blood sample should be collected for further HIV testing. If necessary, HIV nucleic acid testing is performed for further definitive diagnosis. In addition, effective management of suspected HIV/AIDS cases is required to prevent the further spread of HIV/AIDS epidemics.
Our findings revealed that different assays differ in terms of their effectiveness for the differential diagnosis of HIV-indeterminate WB samples. In this regard, it has previously been shown that HIV p24 antigen can be detected earlier than HIV antibodies can (Tsoukas and Bernard 1994); however, the p24 antigen cannot be detected for a prolonged period of time after the acute phase of HIV infection, due to the formation of p24 antigen–antibody complexes. Nevertheless, p24 antigen can subsequently be detected again in blood samples until the advanced stage of HIV infection when the host immune system is severely damaged and fails to produce antibodies. Accordingly, in the present study, our results indicated the low sensitivity (42.59%) of HIV-1 p24 antigen detection. It is thus considered that p24 antigen detection is not a satisfactory approach for the differential diagnosis of indeterminate results, and negative results obtained based on p24 antigen detection cannot completely exclude the occurrence of HIV infection (Dodd and Stramer 2000; WHO 2006). However, a positive detection of p24 antigen, assessed in conjunction with clinical symptoms, may be used to indirectly infer the course of HIV/AIDS (early or advanced stage) and the severity of damage caused to the immune system.
In response to the recent advances in nucleic acid detection, the US Centers for Disease Control and Prevention in June 2014 updated the guidelines for laboratory testing for the diagnosis of HIV infections, and nucleic acid detection has been used as a laboratory test for screening suspected or indeterminate samples (CDC 2014). In addition, HIV nucleic acid testing has been employed as a supplementary test for the diagnosis of HIV-1 infections, in the identification of samples with reactions in antibody re-tests and HIV antibody indeterminate samples, and in the diagnosis of HIV infection at early and advanced stages, in accordance with the 2015 revised National Guidelines for Detection of HIV/AIDS of China (MOH 2016).
Our data indicated that both qualitative and quantitative HIV nucleic acid tests showed a high specificity and sensitivity and could be used as effective tools for the differential diagnosis of HIV-indeterminate samples. However, we obtained a few false-positive and false-negative results in HIV testing, with the false-positive rate of quantitative HIV nucleic acid testing being 9.68%, which may be attributable to experimental contamination of the qPCR assay mix or non-specific primer amplification. Moreover, erroneous results may be associated with nonstandard experimental procedures. Further analyses revealed that the HIV viral load of false-positive samples was lower than 5000 copies/mL, which is similar to values reported in previous studies (Kakaiya et al. 2011; Schmidt et al. 2010). According to the 2015 revised National Guidelines for Detection of HIV/AIDS, those samples with a HIV viral load of below 5000 copies/mL require repeated testing (MOH 2016). However, in the case of the present study, we were unable to obtain blood samples from some subjects for monitoring the HIV viral load, and the HIV antibody re-test results obtained through telephone followup were used to identify the follow-up outcomes. Therefore, the possibility of false-positive results attributable to factors associated with the HIV WB assay cannot be excluded. Since HIV nucleic acid qualitative testing requires amplification of HIV Env, Gag, and Pol gene segments, when at least two segments of these three HIV-1 genes are amplified, the sample is considered to be HIV-1 nucleic acid positive. Therefore, the likelihood of falsepositive results obtained by qualitative HIV nucleic acid testing is lower than that obtained using quantitative testing. Those samples determined to be positive based on quantitative HIV nucleic acid testing, particularly low-copy samples, should receive follow-up HIV re-testing. If the test results for two blood samples are both positive at different time points, the real infection should be diagnosed together with clinical and epidemiological data, or parallel testing should be performed with qualitative nucleic acid testing to avoid misdiagnosis.
In this study, there were a few samples for which we obtained false-negative results. This may be explained by inappropriate storage, mismatching between virus variation and the currently used primers, or drug self-administration that leads to the inhibition of virus in the plasma (Patel et al. 2010; Wesolowski et al. 2011). Therefore, the HIV nucleic acid test results should be carefully assessed. If the nucleic acid test results are negative, but clinical or epidemiological data strongly support the likelihood of HIV infection, fresh blood samples should be collected or different test kits should be considered for HIV re-testing. Additionally, details should be obtained regarding the administration of any antiviral agents. If patients have undergone antiretroviral therapy, and the plasma virus load is undetectable, anticoagulated whole blood samples should be collected for the extraction of proviral DNA from lymphocytes for subsequent qualitative HIV nucleic acid testing.
In summary, the findings of the present study indicate that the proportion of HIV-indeterminate WB results is lower in southeastern China, and that the proportion tends to vary among different subject populations. Understanding the origin of HIV-indeterminate samples and WB band patterns may facilitate the prediction of HIV infection. When used in conjunction with the corresponding epidemiological data, this information can assist practitioners in proposing targeted medical follow-up strategies. In addition, we demonstrated that both qualitative and quantitative HIV nucleic acid testing can be effective for the differential diagnosis of HIV-indeterminate samples, which can aid in the timely identification of HIV infections, and subsequently the implementation of appropriate interventions and therapy, thereby preventing or reducing the spread and transmission of HIV. Nevertheless, non-HIV infections should be excluded as early as possible, to eliminate the psychological pressure and minimize problems in the lives and work of those affected.
-
We would like to thank Drs. Hanhui Ye, Yahong Chen, and Xiaoyan Lin from the Mengchao Hepatobiliary Hospital of Fujian Medical University, and the Centers for Disease Control and Prevention in all cities of Fujian Province, for their kind assistance during the investigation. This study was supported by grants from the Cultivation of Young Talents Project Fund from the Fujian Provincial Health and Family Planning Commission (Grant No. 2015-ZQN-ZD- 11), the Pilot Project of Fujian Provincial Department of Science and Technology (Grant No. 2016Y0010), and the Jiangsu Provincial Project of Invigorating Health Care through Science, Technology and Education and Jiangsu Provincial Medical Youth Talent, the Project of Invigorating Health Care through Science, Technology and Education (Grant No. QNRC2016621).
-
SW, PY and YY designed the experiments. SW, MG and JZ carried out the experiments. SW, MG, WW, XL and YQ analyzed the data. SW, MG and WW wrote the paper. SW and YY checked and finalized the manuscript. All authors read and approved the final manuscript.
-
The authors declare that they have no conflict of interests.
-
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Review Committee of Fujian Municipal Center for Disease Control and Prevention and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants enrolled in the study.














 DownLoad:
DownLoad: