HTML
-
Retroviruses (family Retroviridae) are often associated with severe infectious diseases. They can occasionally infect germ cells and colonize germlines, and thus are capable of vertical transmission in a Mendelian fashion and evolving along with the host. Inherited retroviral sequences are referred to as endogenous retroviruses (ERVs) and are commonly found in vertebrates (Stoye 2012). A typical ERV possesses two long terminal repeats (LTRs) and retroviral gag, pol, and env genes (Fig. 1A), representing past infection of its exogenous ancestor. ERVs can be classified into three major groups: Class Ⅰ, Gammaretrovirus-like; Class Ⅱ, Betaretrovirus-like; and Class Ⅲ, Spumaretrovirus-like (Fig. 1B). The human genome consists of a considerable number of retroviral elements, called human endogenous retroviruses (HERVs), and the most widely distributed is HERV-K (also called HML-2). HERV-K has a typical genome length ranging from 7 to 11 kb. Few proviruses still possess intact open reading frames (ORFs) that can be transcriptionally active, but they do produce viral proteins under certain circumstances (Subramanian et al. 2011).
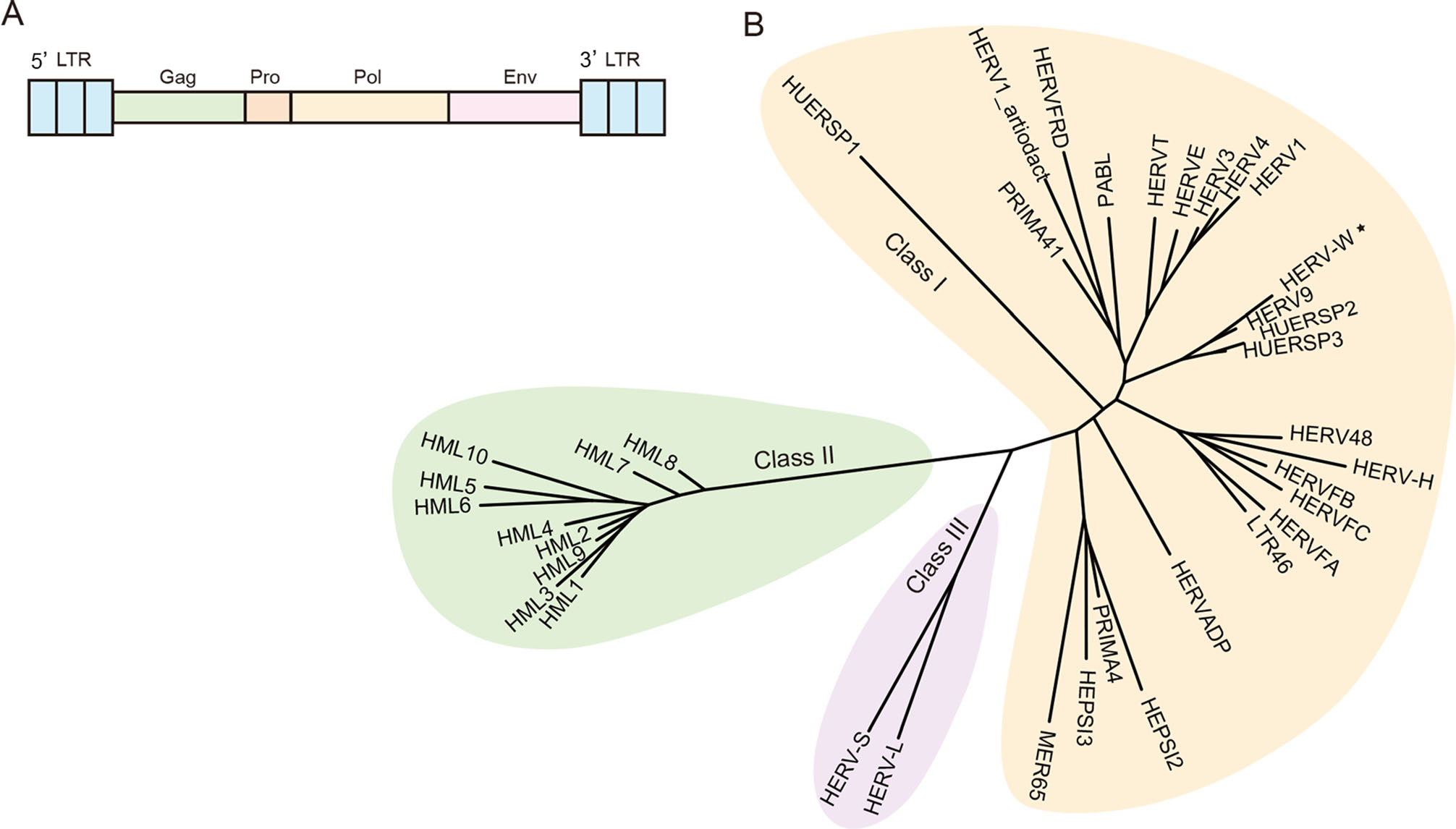
Figure 1. A A brief sketch map of endogenous retrovirus. An intact ERV includes gag, pro, pol, env genes and two long terminal repeats. B Current phylogeny of representative HERV clade. HERVs are divided into three groups, Class Ⅰ (Gamma-like), Class Ⅱ (Beta-like), Class Ⅲ (Spuma-like). The classification of HERVs was constructed based on the article Classification and characterization of human endogenous retroviruses; mosaic forms are common (Vargiu et al. 2016). Reference POL proteins also come from this paper. Phylogenetic tree was constructed by using IQ-TREE 2, with ultrafast bootstrap 1000 (Hoang et al. 2018; Minh et al. 2020). Asterisk indicates HERV-W belongs to Class Ⅰ using pol gene to construct phylogenetic tree, but belongs to Class Ⅱ (D-type-related retrovirus) if using ENV protein motif to construct phylogenetic tree (Lin et al. 2010; Henzy and Johnson 2013).
Most HERVs have lost coding capacity during longterm selection and cannot generate new infectious viral particles. However, retroviral products can be co-opted to play biological roles and thus benefit the host. For example, the HERV-W family can produce the envelope protein Syncytin-1, which is essential for embryonal implantation and for the development of the placenta syncitiotrophoblast (Mi et al. 2000). HERVs also regulate the development of the human embryo at the epigenetic level (Hanna et al. 2019). HERV-K can produce transcripts in various tissues, and it can also be detected in some types of cancer, such as melanomas (Katsura and Asai 2019), germ cell cancer, and ovarian cancer (Ruprecht et al. 2008). Although there is no direct causality between HERVs and cancer, aberrant transcription and translation of HERVs has been observed in cancer patients. HERVs are also related to neurodegenerative diseases, and some HERV-K envelope proteins were shown to play a role in the degeneration of neurons, e.g., in amyotrophic lateral sclerosis (ALS) (Douville et al. 2011; Eirew et al. 2015; Li et al. 2015). It seems that the dysregulation of HERV transcripts may have a significant effect on human health.
Some HERV transcripts or viral proteins are hypothesized to be signals of elevated disease state or viral infection. A research team proposed that multiple sclerosis associated retrovirus envelope protein (MSRV-Env) (an inflammatory protein encoded by HERV-W), which could be detected significantly in patients with chronic inflammatory demyelinating polyradiculoneuropathies (CIDP) in the early stage. By using a neutralizing antibody targeting MSRV-Env, CIDP patients with expression of MSRV-Env can be diagnosed before lesions have much evolved (Faucard et al. 2016). Epigenomic modification of HERVs also has the potential to be developed to create biomedicine markers for the early diagnosis of cancer (Hu et al. 2017). Here we thus conclude and propose that HERVs can be used as biomarkers for extensive medical research.
-
Syncytin-1, which is encoded by HERV-W, plays a significant role in mediating the formation of the placenta during embryogenesis. There is a single layer cell of trophoblasts when the embryo enters the uterus. During implantation, some trophoblast cells will fuse to generate coenocytes called syncytiotrophoblasts (Gude et al. 2004). The syncytiotrophoblast is the key part of the placenta, which separates and connects the maternal body and fetus and releases hormones. Researchers have confirmed that Syncytin-1 is a crucial molecule for driving the trophoblast fusion that generates syncytiotrophoblasts (Huang et al. 2014).
Although early studies showed that multiple sclerosis (MS) was mainly caused by abnormal adaptive T cell mediation, increasing evidence indicates that the aberrant high expression of HERV-W ENV also leads to the occurrence of the disease. Coupled to a vital function during development, HERV-W glycoproteins are strongly correlated with neuroinflammation in MS. Research on MS patients elucidated that another HERV-W envelope protein is detected in some tissues and cells by specific RNA with primers differentiating Syncytin from MS-associated HERV-W (Mameli et al. 2009; Perron et al. 2012). This HERV-W ENV protein from MS was previously named MSRV-Env and is now designated as pHERV-W Env, to specify its pathological origin (Kremer et al. 2019). Envelope proteins were also detected in the brain and peripheral blood in patients. In addition to HERV-W, there are many other HERVs associated with diseases. Neuropsychological disorders such as schizophrenia are closely related to abnormal expression of HERV-W (Aftab et al. 2016), and research on mice indicated that HERV-K could contribute to neuropsychological disorders via the TLR (Toll-like receptor) signal pathway (Kury et al. 2018; Wang et al. 2018a; Dembny et al. 2020), inducing strong cytotoxic T lymphocyte responses or increasing nitric oxide (Tu et al. 2017; Xiao et al. 2017). It was reported that different transcripts from HERV-H, HERV-K, HERV-R, and HERV-S (Fig. 1B) showed aberrant expression in human cancer cell lines (Kury et al. 2018; Zhang et al. 2019).
Defective HERVs also have the ability to regulate adjacent genes. For example, solitary LTRs (solo LTRs) can function as alternative promoters over adjacent genes (Singer et al. 2008). The generation of B cell-derived Hodgkin's lymphoma cells depends on the activity of the colony-stimulating factor 1 receptor (CSF1R). Some evidence indicated that the aberrant activation of the LTR started the transcription of CSF1R because LTR-driven CSF1R transcripts in lymphoma cells could be detected (Lamprecht et al. 2010). Consequently, activated CSF1R could promote B cell-derived Hodgkin's lymphoma. By functioning as alternative promoters or transcription regulators, HERV LTRs apparently have a dual nature, either beneficial or harmful to the host when regulation is disrupted.
DNA methylation is a common process in the regulation of organism development. It is remarkable that so many methylated CpG islands locate at cluster regions of repetitive elements like HERVs in the human genome (Walsh et al. 1998). The demethylation process usually occurs during the process of erasing imprinted genes in embryonic stem cells (Hanna et al. 2019). However, some studies have found that HERV demethylation events occur in certain types of tumors. One example is that the demethylation of 5'-LTR leads to increased HERV-K expression in melanomas (Türkmen et al. 2011); another is that the hypomethylation of the U3 promoter in the LTR region can cause upregulation of HERV, which may activate oncogenesis (Gimenez et al. 2010). The high expression level of HERVs conveys a disease signal, which may keep the human body alert to the process of lesion development (Chiappinelli et al. 2015). It is of note that several studies share the same opinion on the functions of HERV-W Gag and Env proteins in schizophrenia patients via the TLR3 or the TLR4 signaling pathways cascade (Perron et al. 2008; Huang et al. 2011; Wang et al. 2018b; Johansson et al. 2020).
-
HERVs can be activated, even with solitary LTRs, upon chemical or physical changes. Phytohemagglutinin (PHA) treatment for peripheral T cells induces HERV-H expression after 3–4 h. Specific probes for U3 and U5 can detect HERV-H LTR after injection with PHA (Kelleher et al. 1996; Johnston et al. 2001). When exposed to two drugs respectively, caffeine had an ability to activate HERV-W env to make the levels of the relevant transcripts of HERV-W gag higher by activation of HERV-W env promoter, and aspirin could upregulate the expression of HERV-W gag in the human neuroblastoma cells compared with the nontreated group (Liu et al. 2013). Moreover, physical factors, which can change the genome structure and make the genome unstable, can promote the abnormal expression of HERV-R. The up-regulated transcription of HERV-R that also occurs in γ-irradiated normal cells may be related to epigenetic changes caused by radiation-induced histone modifications. There are also some studies suggesting UV radiation may induce HERV expression (Lee et al. 2012).
In addition to chemical and physical factors, in some cases, exogenous viral infections contribute to HERV reactivation. Post-antigen stimulation experiments in MS patients indicated that herpes simplex virus 1 (HSV-1), human herpesvirus 6 (HHV-6), and varicella zoster virus (VZV) encoded specific antigens, which activated HERV envelope protein (Charvet et al. 2018). Another study found that hepatitis B virus X protein (HBx) upregulated mRNA and protein levels of HERV-W ENV in human hepatoma HepG2 cells (Liu et al. 2017). Moreover, it was recorded that influenza virus infection in nonplacental cells could cause Syncytin-1 reactivation (Li et al. 2014). It is a phenomenon of great interest in the field of cancer research because it may partially explain why some viral infections can induce tumors. Also, worth noting is that not all herpes viruses can activate HERVs in patients, such as Epstein–Barr virus (EBV) (Brudek et al. 2007; Mameli et al. 2012, 2013). By comparing the differences in the ability of exogenous retroviruses to induce ERVs, we can better understand the activation mechanism of HERVs.
HIV-1 is an exogenous retrovirus that can induce HERV-K expression in vitro. Cells infected with HIV-1 particles produce higher HERV-K RNA transcripts compared with the control. The replication of HIV-1 in peripheral blood mononuclear cells (PBMCs) promotes the expression of HERV-K and generates several proteins, which are a heavy burden for those cells. In return, increasing HERV-K expression helps to maintain a constant generation of HIV-1 virions. The two viruses seem to complement each other, promoting each other's expression in a positive regulatory way (Contreras-Galindo et al. 2007).
Mutations including histone mutation and LTR mutation promote overexpression of ERV. H3.3 deletion in mouse embryonic stem cells reduces H3K9me3 and results in derepression of adjacent ERV genes (Elsässer et al. 2015). Mutations in 3'-LTR of HERV-W upregulate Syncytin-1 expression in urothelial cell carcinoma (Yu et al. 2014). It shows that the potential of specific agents (such as diseases and immunologic deficiency) under specific circumstances can activate HERVs. In-depth research may provide us with a unique perspective on the relationship between the human body and HERVs and help us discover possible treatments for some specific diseases.
-
Cancers are usually caused by multiple factors. In many common human cancers, transcription and translation traces of HERVs can be detected. This observation indicates a link between HERVs and cancers. It is well known that exogenous retroviruses can insert their genome into the host genome by reverse transcription. The insertion of HERVs in less differentiated cells may lead to the damage of tumor suppressor genes, which promotes the proliferation of cells and the formation of tumors. Nevertheless, some inserted retroviruses can also induce tumor generation after years of peaceful coexistence during evolution (Kassiotis 2014). As mentioned above, defective HERVs play an essential role in the regulation of adjacent genes, and occasionally their overexpression leads to cell proliferation. The products of two HERV-K (HML2) env genes, the Np9 and Rec proteins, are known for being frequently found in testicular germ cell tumors (GCTs), but not in healthy cells (Ruprecht et al. 2008). Patients with GCT can produce enough serum antibodies targeting Rec proteins, whose transcripts originated from the env gene of HERV-K sequences. Mice with induced expression of HERV-K Rec proteins develop carcinoma in situ with human-sourced HERV-K, which will develop into seminoma in human beings (Galli et al. 2005). Overexpression of Syncytin-1 happens in breast carcinoma cells and urothelial carcinoma cells (Yu et al. 2014; Chignola et al. 2019). Methylation of HERV-W has a significant decline, and the expression level is elevated in ovarian tumor tissue (Menendez et al. 2004).
Renal cell carcinoma (RCC) is a common malignant kidney tumor. Hypoxia-inducible transcription factor (HIF) plays a cardinal role in stimulating POU5F1 expression in RCC oncogenesis (Nanus and Gudas 2016). Examination of the POU5F1 genomic locus in some RCC cells indicated the existence of an HIF-pathway-responsive promoter embedded within an endogenous retroviral LTR, which is located at the transcriptional start site of PSOR1C3, upstream of POU5F1. Transcriptome analysis shows a novel POU5F1 transcript isoform induced from the promoter embedded in the LTR, which reads through PSOR1C3 into POU5F1 (Siebenthall et al. 2019). Abnormal lengthy transcripts accumulate in tumor tissues and change the tumor immune microenvironment, which promotes tumor development (Smith et al. 2018).
Given the relationship between HERVs and cancers, the production of HERVs can be seen as a biomarker of specific cancers, which is useful for improving diagnostics and therapy-treatment selection. Because of the inaccuracy and slowness of traditional cancer diagnosis methods, such as imaging technology and pathological examinations, it is essential to find faster and more precise methods. As lung cancer is a major cause of death throughout the world, it would be of great significance to find a precise way to achieve early diagnosis of lung cancer. A recent study using quantitative gene expression analysis reported a positive and significant pairwise correlation between the expression of the HERV env gene and different lung tumors. Four env gene transcripts of HERVs, namely, HERV-R, HERV-H, HERV-K, and HERV-P, are significantly increased in the peripheral blood of lung cancer patients (Zare et al. 2018). Abnormal transcription means aberrant internal milieu regulation, which indicates a potential ability to gain cancerization. With evidence that the role of HERVs correlates with the tumor microenvironment, the expression of HERVs could serve as biomarkers for cancer diagnosis and immunotherapy.
In addition to the application of HERVs in the diagnosis of lung cancer and RCC, advanced prostate cancer can be diagnosed by the expression of the gag gene of ERV. Compared with healthy people, prostate cancer patients show a much higher expression of gag of HERV-K. In some cases, there are antibodies against the Gag protein of HERV-K in the serum of patients despite no detectable gag protein being found. Interestingly, HERV-K antibodies are found not only in advanced prostate cancer but also in melanoma, breast cancer, and ovarian cancer (Reis et al. 2013). Fortunately, monoclonal antibodies targeting the HERV-K env protein can block the proliferation of human breast cancer cells in vitro and inhibit tumor growth in immunodeficient mice grafted with human tumors (Cegolon et al. 2013).
HERVs and Diseases
Activation of HERVs
HERVs in Tumorigenesis
-
The abnormal activation and expression of HERVs correlates with many diseases, such as cancers, immunologic deficiency diseases, and neurodegenerative diseases. Demethylation on HERV loci is also widely observed in different types of cancers. With the advancement of sequencing technology and the continuous development of various omics, novel HERVs are likely to be found in humans and related primate species. Activation of HERVs not only shapes the transcription network, but also assists other viruses to stimulate host antiviral immunity. Those modern consequences of past viral infections are correlated with some human diseases, ranging from cancers to neurological diseases, autoimmune diseases, and some other epigenetic diseases. The application of HERVs as biomarkers in the era of biomedicine is approaching its coming of age.
-
This study was supported by the National Science and Technology Major Project (Grant No. 2018ZX10301101), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA13010500) and the CAS Pioneer Hundred Talents Program to JC.
-
The authors declare that they have no conflict of interest.
-
This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: