HTML
-
Influenza is an acute respiratory infectious disease caused by infection with influenza virus. Annual seasonal influ-enza epidemics and occasional emerging pandemics, such as 2009 swine influenza and sporadic emerging highly pathogenic avian influenza viruses (H5N1 and H7N9), became a serious health burden. Influenza A virus (IAV) belongs to Orthomyxoviridae family, which is a single-stranded, negative-sense RNA enveloped virus consisting of eight segmented genomes (Shaw and Stertz 2018). As an obligate pathogen encoding limited proteins, the influenza virus relies on host proteins and machinery to harbor infection and complete its life cycle (Krammer and Palese 2015).
Promyelocytic leukemia protein (PML), also known as MYL/RNF71/PP8675/TRIM19, is the organizer of the PML nucleus bodies (PML-NBs), which are dynamic cel-lular structures that contain many transiently and perma-nently localized proteins (Scherer and Stamminger 2016). On the basis of the splicing of a single gene, PML is divided into seven isoforms, PML I–VII (Nisole et al. 2013). All PML isoforms share the N-terminal region, which encodes the RBCC motif, while their C-terminal regions show remarkable variability and account for iso-form-specific roles. In normal cells, PML proteins are distributed in discontinuous speckled patterns within the nucleus, known as components of the PML-NBs or PML oncogenic domains (PODs) subnuclear region (Negorev and Maul 2001). A few PML proteins, such as PML VIb and PML VIIb, are localized to the cytoplasm due to lack of the nuclear localization signal (NLS). PML, as the major component of PML-NBs, plays important roles in pro-grammed cell death (Hsu and Kao 2018), cell senescence (Li et al. 2017), transcriptional regulation (Wang et al. 2018), genome stability (Chang et al. 2018), and antiviral defense (Blondel et al. 2010; Li et al. 2009; Alandijany et al. 2018; Chen et al. 2018; Chelbi-Alix et al. 2006; El Asmi et al. 2014).
PML has been demonstrated to display antiviral activi-ties against DNA and RNA viruses in vitro, including vesicular stomatitis virus (VSV) (El Asmi et al. 2014), influenza virus (Li et al. 2009), herpes simplex virus (HSV) (Alandijany et al. 2018), human enterovirus 71 (EV71) (Chen et al. 2018) and rabies virus (Chelbi-Alix et al. 2006). Moreover, PML knockout mice were more sensitive to lymphocytic choriomeningitis virus (LCMV) (Kentsis et al. 2001) and VSV (El Asmi et al. 2014), which sug-gested that PML also had antiviral activity in vivo. It is well known that the replication of virus depends on a variety of host proteins in the nucleus. As an important subnuclear structure, PML-NBs are involved in the innate immune response of cells against viral invasion or the cellular antiviral response mediated by IFN. The relevant proteins PML, Sp100, PA28 and ISG20 were directedly induced by IFN and PML depletion reduced the ability of IFNs to resist viral infection (Chelbi-Alix et al. 1995), suggesting that PML-NBs could play an important role in IFN response. Although the function of PML NBs is not fully understood, some results show that they are increasingly recognized to be preferential targets for viral infections. Studies have shown that PML could play an important role in the antiviral action of IFNs via a higher activation of IRF3 (El Asmi et al. 2014), however, viruses have evolved a dual role against IFN-induced antiviral action. Viruses resist the antiviral activity by regulating PML expression and/or localization on nuclear bodies (Maul et al. 1993) and by inhibiting IFN signaling way (Everett and Chelbi-Alix 2007). Although the inhibitory activity of PML against DNA and RNA viruses has been partially eluci-dated, the mechanism of PML against influenza virus is still unclear.
FBXW7 (also known as FBW7/SEMO/hAgo/hCDC4) is one of the components of the Skp1-Cul1-F-box (SCF) ubiquitin-ligase SCFFBXW7. SCFFBXW7 acts as a node for proteasomal degradation and plays an important role in cell proliferation and differentiation, lipid metabolism, and the maintenance of stem cell homeostasis (Welcker and Clurman 2008), including KLF2 (Wang et al. 2013), cyclin E2 (Moberg et al. 2001), c-Myc (Yada et al. 2004), Mcl-1 (Inuzuka et al. 2011) and Notch (Tsunematsu et al. 2004). FBXW7-deficient mice are more sensitive to RSV, VSV and influenza A virus infection and decreases type Ⅰ interferon production. In addition, Song et al. showed that FBXW7 degraded the phosphorylated Src homology-2 domain-containing phosphatase 2 (SHP2), which nega-tively regulated the RIG-I/IRF3 signaling pathway and innate antiviral immunity (Song et al. 2017). However, the relationship between PML and FBXW7 during influenza virus infection has not been reported.
In this study, we confirmed the effect of PML against influenza virus and mainly explored the anti-influenza virus mechanism of PML for the first time. It provides a new idea for the discovery of anti-influenza virus targets and a theoretical basis for revealing the function of PML.
-
Madin-Darby canine kidney (MDCK) cells, human lung adenocarcinoma epithelial cells (A549) and human embryonic kidney 293 T/17 cells (HEK293T/17) were obtained from American Type Culture Collection (ATCC); RAW264.7 cells were purchased from Cell Bank of the Chinese Academy of Sciences (Shanghai, China). MDCK cells were grown in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), antibi-otics (100 U/mL penicillin and 100 μg/mL streptomycin), and 1% MEM nonessential amino acid solution. A549 cells were cultured in F12K medium supplemented with 10% FBS and antibiotics. Human embryonic kidney 293 T/17 (HEK293T/17), and RAW264.7 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supple-mented with 10% FBS and antibiotics.
Influenza virus A/Fort Monmouth/1/1947 (FM1, H1N1) was purchased from the ATCC (VR-97), A/HanFang/359/ 95 (HanFang, H3N2) strain was kindly provided by Pro-fessor Yuelong Shu at the Institute for Viral Disease Control and Prevention, China Centers for Disease Control and Prevention, Beijing, China. Viral stocks were prepared by propagating them in 10-day-old embryonated Chicken eggs for 48 h.
-
Bafilomycin A1 (Baf A1) was purchased from Sangon Biotech (Shanghai, China). Cycloheximide (CHX) was purchased from MedChemExpress (NJ, USA). MG132 was obtained from Selleckchem (TX, USA).
The plasmids of pcDNA3.1-Myc-PMLIV, pcDNA3.1-Flag-Ub, and pcDNA3.1-Myc-FBXW7 were provided by Professor Zhuowei Hu from the institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). pCMV3-FBXW7-His was purchased from Sinobiological Inc (Beijing, China). A cDNA encoding the full-length human SHP2 were cloned into the pcDNA3.1(+) vector. All constructs were confirmed by sequencing. PML-siRNA (si-PML) and Control-siRNA (si-NC) were purchased from Santa Cruz (sc-36284, CA, USA).
Antibodies against influenza A M2 (sc-32238) and influenza A NS1 (sc-130568) were obtained from Santa Cruz (CA, USA). The antibody against PML (NB100-59,787) was from Novus Biologicals (Colorado, USA). The antibody against FBXW7 (H5081-3D-1) was purchased from Abnova (Taipei, Taiwan). Antibodies against SHP2 (#3397), RIG-I (#3743), HA-Tag (#2367), Myc-Tag (#2276), b-Actin (#3700), Tubulin (#2148), Alexa Fluor 488-conjugated goat anti-mouse (#4408) or rabbit (#4412) IgG, and Alexa Fluor 647-conjugated goat anti-mouse (#4410) or rabbit (#4414) IgG were purchased from CST (Cell Signaling Technologies, USA). Antibodies against Histone H3 (17,168-1-AP), His-Tag (66,005-1-Ig), and Flag-Tag (66,008-2-Ig) were obtained from the Proteintech Group (IL, USA). HRP-conjugated secondary antibodies against mouse (HS201-01) or rabbit (HS101-01) IgG were purchased from TransGen (Beijing, China).
-
A549 cells in a 6-well plate were transfected with the indicated plasmids. The cells were infected with IAV at a multiplicity of infection (MOI) of 0.2 at 24 h post-trans-fection. At 24 h post infection, cells were frozen and melted thrice, and the supernatant was harvested after centrifugation for removing cell debris. The virus titers were performed by measuring TCID50. Confluent mono-layers of MDCK cells were infected through tenfold seri-ally diluted supernatant in 96-well plates. The medium was changed 2 h after virus infection. The cytopathic effect (CPE) of cells was observed at 48 h, and TCID50 was calculated by Reed & Muench method.
-
Protein extracts (10 μg) were subjected to electrophoresis with 10% polyacrylamide gel and were blotted onto PVDF membranes (Millipore Corp.). The membrane was incu-bated with primary antibody overnight at 4 ℃, followed by incubation with HRP-conjugated rabbit anti-mouse or goat anti-rabbit IgG. The signal was detected with an ECL kit.
-
HEK293T/17 cells were transfected with the indicated plasmids for 48 h. Cells were collected and lysed in M-PER mammalian protein extraction reagent that contained halt protease inhibitor cocktail (MA, USA) for 30 min at 4 ℃ and then centrifuged at 12,000 ×g for 20 min. The supernatants were incubated with 4 μg of the indicated antibody overnight. Agarose beads were added to the lysate for 3 h and then washed with washing buffer. The samples were boiled for 5 min and analyzed by SDS-PAGE and immunoblotting.
-
For intracellular viral RNA quantification, the total RNA was extracted from the cells by using RNeasy Mini Kit (Qiagen) according to the manufacturer's instruction. Then, quantitative reverse transcription PCR (qRT-PCR) was performed using the TransScriptII Green One-Step qRT-PCR SuperMix Kit (TransGen Biotech Co., Ltd. Beijing, China) according to the protocol. The sequences of the primers are depicted in Supplementary Table S1.
-
A549 cells were grown in coverslips in 12-well plates overnight. Cells were incubated for 2 h with the influenza virus at 37 ℃ in 5% CO2 for adsorption. After 2 h, inoculum was decanted, and infected cells were supple-mented with maintenance medium. After 18 h, A549 cells were washed three times with PBS and then fixed for 10 min with 4% paraformaldehyde. The cells were perme-abilized in 0.5% Triton X-100 for 15 min. The slides were then blocked with 3% BSA for 1 h at room temperature and further incubated with the influenza virus M2 antibody overnight at 4 ℃. After washing three times with 0.1% TBS-Tween, cells were incubated with Alexa Fluor 488 anti-mouse IgG for 1 h. The nucleus was stained with DAPI (Beyotime, Shanghai). Photos were taken with an Olympus TH4-200 microscope.
For colocalization studies with FBXW7-His and PML, fixed A549 cells were permeabilized in 0.5% Triton X-100 for 15 min and blocked with 3% BSA for 1 h at room temperature. The cells were incubated with rabbit anti-His-tag and mouse anti-PML antibody overnight at 4 ℃, rinsed with TBST, and stained with Alexa Fluor 647 anti-rabbit IgG and Alex Fluor 488 anti-mouse IgG for 1 h. The slides were mounted with DAPI. Images were acquired with a Zeiss LSM 710 microscope.
-
All data were analyzed by the SPSS 19.0 software, com-paring with groups using one-way ANOVA, and the two independent groups were analyzed via Student's t-test. Data are showed as means ± SD. Statistical differences were considered significant at *P < 0.05 and **P < 0.01.
Cells and Viruses
Compounds, Plasmids, and Antibodies
IAV Infection and Virus Titer Determination
Western Blot
Immunoprecipitation
Quantitative Real-time PCR (qRT-PCR)
Indirect Immunofluorescence Assay
Statistical Analysis
-
To determine whether IAV infection impacted PML expression, A549 cells were infected with at various mul-tiplicity of IAV (MOI = 2, 0.2, 0.02, and 0.002), and the protein and mRNA levels were determined by Western blot and qRT-PCR. As shown in Fig. 1A and 1B, both the protein and mRNA levels of PML were upregulated after IAV infection. Moreover, the protein and mRNA levels of PML were significantly upregulated at 8 h post-infection (pi) and 24 h pi (Fig. 1C and 1D). The results suggest that the expression of PML was significantly induced by IAV infection in a time- and dose-dependent manner. Previous studies had shown that the PML-NBs changed differently under different cell types, cell cycles, or external stimula-tions (Batty et al. 2012). To determine whether IAV infection altered PML nuclear bodies, we performed immunofluorescent staining experiment. As shown in Fig. 1E, the number of PML nuclear bodies (PML-NBs) increased in IAV-infected cells compared with uninfected cells at 24 h pi. These data indicate that IAV infection upregulates the expression of PML and increases the number of PML-NBs in A549 cells.
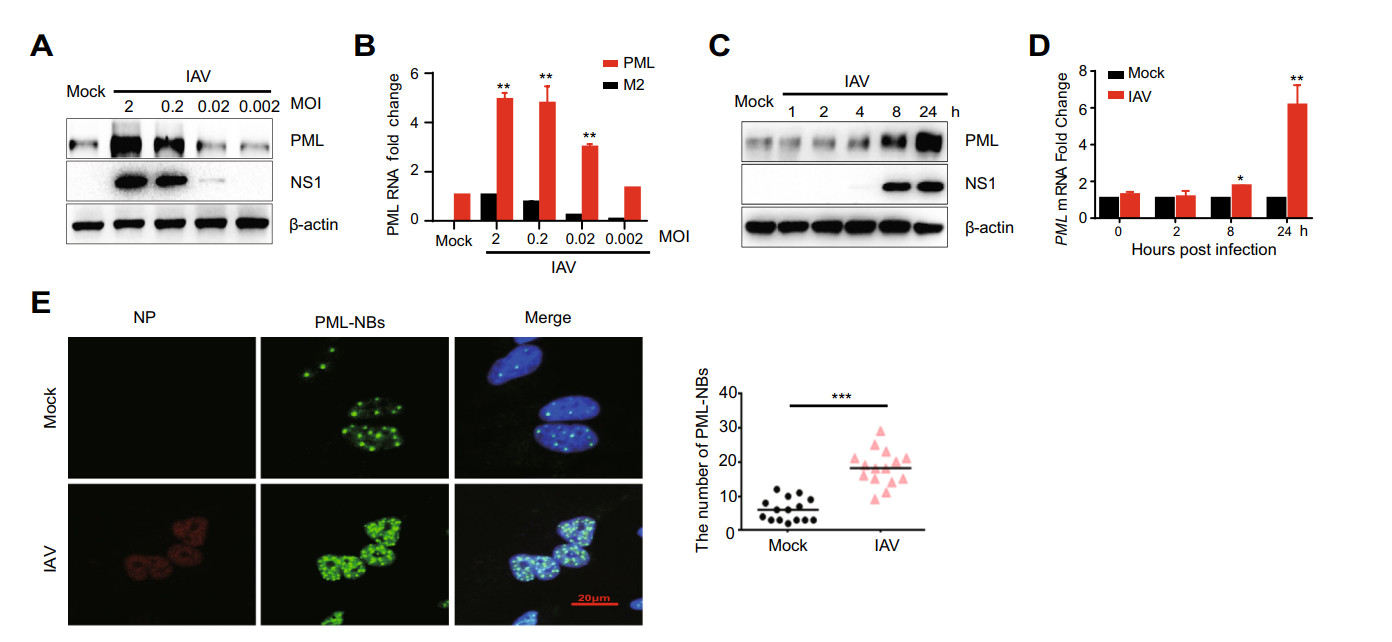
Figure 1. IAV infection upregulates PML expression in A549 cells. A549 cells were infected with IAV (H1N1/FM1, MOI = 2, 0.2, 0.02, 0.002) for 2 h. A After 24 hpi, the protein levels of IAV NS1 and PML were analyzed by Western blot(Without special instructions, the strain of H1N1/FM1was used in all experiments.). B RNA from cells was extracted at 12 hpi and was subjected to qRT-PCR analysis to determine the expression of PML and M2. C A549 cells were infected with IAV (MOI = 0.2). After 1, 2, 4, 8, and 24 h, the protein levels of IAV NS1 and PML were analyzed by Western blot. D PML mRNA was examined by qRT-PCR at 0, 2, 8, and 24 hpi. The experiments were performed in triplicate. Each value represents means ± SD. *P < 0.05; **P < 0.01 versus 0 h group. E A549 cells were infected with IAV, and fixed at 24 hpi (MOI = 0.2) and subjected to immunofluorescence to detect NP (red) and PML nuclear bodies (PML-NBs, green). Nuclei stained by DAPI are shown in blue. Scale bar is 20 μm. The number of PML-NBs was counted in a single cell (n = 15).
-
To evaluate the effects of PML on IAV replication, A549 cells were transfected with Myc-PML plasmid and then infected with IAV. As shown in Fig. 2A, 2B and 2C, compared to the control cells, IAV NS1 and M2 protein level (Fig. 2A and 2C) and RNA level (Fig. 2B) signifi-cantly decreased. In addition, we observed that the viral titers dose-dependently decreased in the cells transfected with external PML (Fig. 2D). In addition, with the expression of external Myc-PML in the A549 cells, IAV replication significantly decreased. To investigate whether reducing the expression of PML can enhance IAV repli-cation, A549 cells were transfected with si-PML or si-NC. Indeed, knockdown of PML enhanced the protein and mRNA levels of IAV (Fig. 2E–2G). For the influenza A virus H3N2 subtype, overexpression of PML in A549 cells inhibited the replication of influenza virus, and knockdown of PML showed higher susceptibility to influenza virus (Fig. 2H and 2I). We further confirmed that virus titer was significantly increased in the PML knockdown A549 cells compared with those in the Si-NC group (Fig. 2J). Taken together, these data indicate that PML inhibits IAV replication.
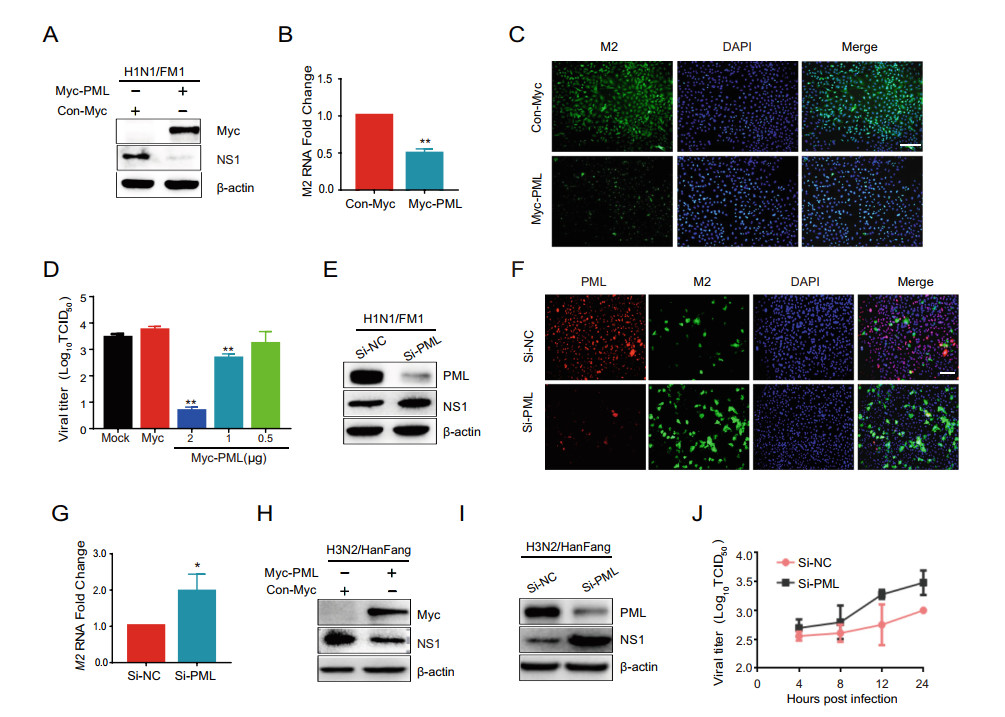
Figure 2. PML inhibits IAV replication in A549 cells. A549 cells transfected with Myc-PML plasmid (1 μg) or Myc vector (1 μg) using lipofectamine 3000 reagent for 24 h. Then, the cells were infected with IAV (MOI = 0.2) for 2 h. A After 24 hpi, PML and IAV NS1 protein expression was analyzed by Western blot. B The total RNA was extracted at 8 hpi (MOI = 0.2) and then subjected to qRT-PCR to analyze the IAV M2 RNA level. C A549 cells were fixed at 24 hpi and then subjected to immunofluorescence to detect IAV M2 (green). Nuclei stained by DAPI are shown in blue. Scale bar is 100 μm. D After transfection with Myc-PML plasmid (2, 1, 0.5 μg) or Myc vector (1 μg) for 24 h, A549 cells were infected with IAV for 2 h, and frozen and melted thrice at 24 hpi. Virus from the supernatant was harvested and viral titers were quantified by measures for TCID50. Results were presented as log10 values of the mean viral load ± SD. E–G A549 cells were infected with IAV (MOI = 0.2) for 2 h after transfection of si-PML (20 nmol/L) and si-NC (20 nmol/L) for 24 h. After 24 hpi, PML and IAV NS1 protein expression were analyzed by western blot and immunofluorescence (E and F). The cellular RNA was extracted at 8 hpi (MOI = 0.2) and then subjected to qRT-PCR to analyze the IAV M2 RNA level (G). H-I A549 cells transfected with Myc-PML plasmid (1 μg) and Myc vector (1 μg) or si-PML (20 nmol/L) and si-NC (20 nmol/L) for 24 h were infected with IAV (H3N2/ HanFang, MOI = 0.2) for 2 h. PML and IAV NS1 protein expression was analyzed by Western blot at 24 hpi. J After transfection with si-PML (20 nmol/L) and si-NC (20 nmol/L) for 24 h, A549 cells were infected with influenza virus at MOI of 2. Virus titer was determined by CPE assay with MDCK cells. Results were expressed as log10 values of the mean viral load ± SD. The experiments were performed in triplicate, *P < 0.05; **P < 0.01 versus con group.
-
As a host limiting factor, PML exhibits antiviral activity against DNA viruses and RNA viruses through a variety of mechanisms. Our results shows that PML has a positive inhibitory effect on IAV replication, which is consistent with previous report (Li et al. 2009). Previous report had suggested that FBXW7, as an E3 ubiquitin ligase, played an important role in the natural immunity against VSV or influenza A virus (Song et al. 2017). Therefore, we spec-ulate that PML may affect IAV replication by regulating FBXW7. First, the interaction between PML and FBXW7 needs to be confirmed. Immunofluorescence imaging showed that the colocalization of PML and FBXW7 in A549 cells (Fig. 3A). Co-IP assay also showed that PML interacted with FBXW7 in PML and FBXW7 exogenous expressed HEK293T/17 cells (Fig. 3B). We also examined whether Myc-PML or His-FBXW7 could interact with endogenous PML or FBXW7 in the context of IAV-infected cells. The forward and inverse Co-IP results also confirmed that PML interacted with FBXW7 (Fig. 3C and 3D) in IAV-infected cells. Previous study reported that FBXW7 could translocate from nucleus into cytoplasm and stabilize RIG-I during virus infection, however, PML IV protein is mainly located in the nucleus. To confirm the location of interaction between PML and FBXW7, we utilized Co-IP assay to observe the interaction along with IAV infection in cytoplasm and nucleus separately. Inter-estingly, we found that PML both interacted with FBXW7 in cytoplasm and nuclear (Fig. 3E), which inspired us to identify the potential function of PML in FBXW7-trig-gered antiviral innate response.

Figure 3. PML interacts with FBXW7. A A549 cells transfected with His-FBXW7 for 24 h were infected with IAV (MOI = 0.2) for 2 h. After 36 hpi, A549 cells were fixed and subjected to immunofluo-rescence to detect PML (green) and His-Tag (red). Nuclei stained by DAPI were shown in blue. Scale bar is 20 μm. B HEK293T/17 cells were co-transfected with His-FBXW7 and Myc-PML plasmids for 36 h. The cell lysates were immunoprecipitated with anti-Myc beads and immunoblotted with anti-His antibody. C and D HEK293T/17 cells were transfected with Myc-PML and FBXW7 or Myc-FBXW7 and PML plasmids for 24 h. Coimmunoprecipitation and immunoblot analysis were conducted on HEK293T/17 cells infected with IAV at 24 hpi. E HEK293T/17 cells transfected with His-FBXW7 and Myc-PML for 24 h, then infected with IAV for 8 h. The cells are collected and then separated into nuclei and cytoplasm. The cell lysates were immunoprecipitated with anti-Myc beads in cytoplasm and nucleus separately.
-
Since PML interacted with FBXW7 in cytoplasm, we suspected whether PML played an anti-IAV role by affecting the cytoplasmic FBXW7. Next, we assessed the effect of PML overexpression or knockdown on FBXW7. The effect of overexpression of PML on FBXW7 tran-scription was detected and there was no significant change in the mRNA level of FBXW7 after overexpression of PML (Fig. 4A). However, overexpression of PML increased the protein level of FBXW7, whereas knock-down of PML significantly inhibited FBXW7 protein expression compared with si-NC (Fig. 4B and 4C). Since PML did not affect FBXW7 transcription, but upregulated FBXW7 protein levels, this suggested that PML may reg-ulate the degradation of FBXW7. Ubiquitination is an important form of posttranslational protein modification, which is involved in the regulation of protein function and maintenance of cell life activities. Therefore, we investi-gated whether PML regulated the ubiquitylation of FBXW7 protein. Overexpression of PML inhibited the ubiquitylation of FBXW7 and prolonged the half-life of exogenous FBXW7 protein degradation (Fig. 4D and 4E). These results suggested that PML enhanced the protein level of FBXW7 by sustaining the stability of FBXW7. To determine which form of the polyubiquitin chains of FBXW7 was catalyzed by PML, we co-transfected HA-K48R Ub, HA-K63R Ub, Myc-PML, and His-FBXW7 plasmids into cells and then detected the polyubiquitination of FBXW7. Results showed that K48R-linked ubiquitina-tion of FBXW7 did not change, but K63R-linked ubiqui-tination of FBXW7 decreased in overexpressing PML cells (Fig. 4F). These data indicated that PML mediated K48-linked ubiquitination of FBXW7.
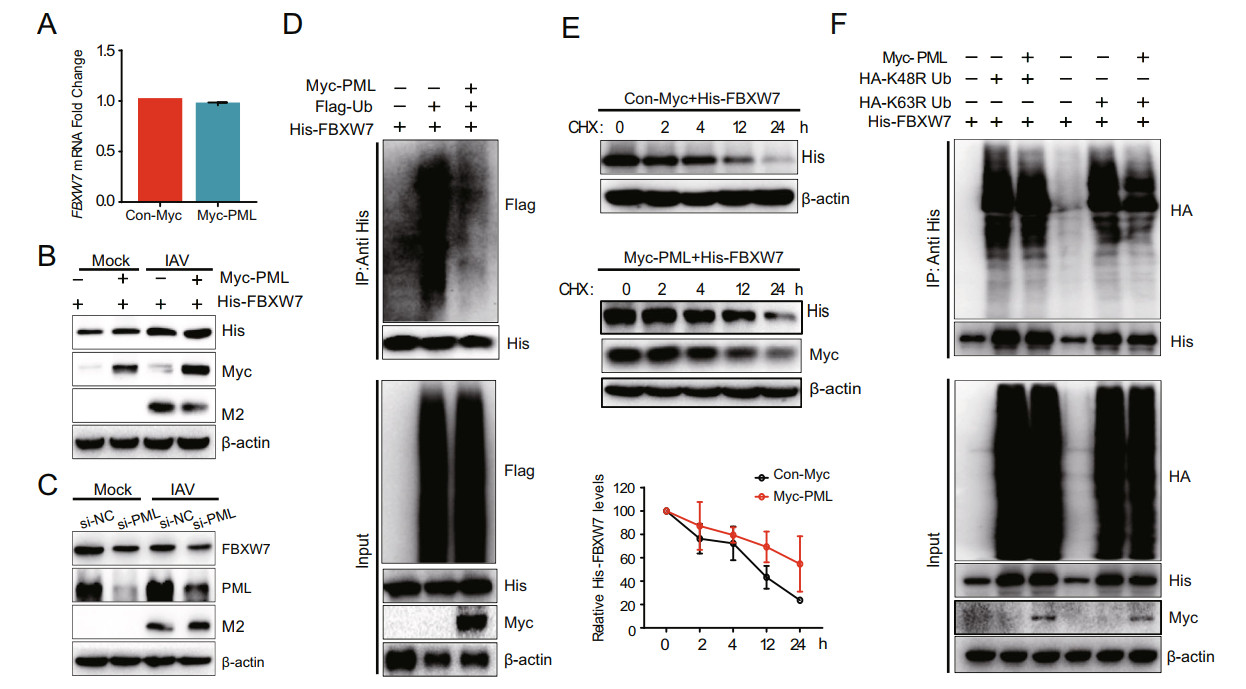
Figure 4. Effects of PML on FBXW7 in A549 cells. A A549 cells transfected with Myc-PML plasmid (1 μg) or Myc vector (1 μg) for 24 h were infected with IAV (MOI = 0.2). The total RNA was extracted at 12 hpi (MOI = 0.2) and then subjected to qRT-PCR to analyze FBXW7 mRNA level. B A549 cells co-transfected with Myc-PML plasmid (1 μg) and His-FBXW7 (1 μg) or with an empty vector were infected with IAV (MOI = 0.2) for 24 h. The expressions of His-FBXW7, Myc-PML, and IAV M2 protein were analyzed by western blot. C A549 cells transfected with si-PML (20 nmol/L) and si-NC (20 nmol/L) for 24 h were infected with IAV (MOI = 0.2) for 2 h. After 24 hpi, the PML, FBXW7, and IAV M2 protein expressions were determined by Western blot. D HEK293T/17 cells were co-transfected with His-FBXW7, Flag-Ub, and Myc-PML plasmids for 36 h with MG132 treatment for 6 h. The cell lysates were immunoprecipitated with anti-His beads and immunoblotted with anti-Flag antibody. E HEK293T/17 cells were transfected with His-FBXW7 and Myc-PML for 24 h and incubated with cycloheximide (CHX, 10 μg/mL) for indicated times. F HEK293T/17 cells were co-transfected with His-FBXW7, HA-K48R Ub, HA-K63R Ub, and Myc-PML plasmids for 36 h with MG132 treatment for 6 h. The cell lysates were immunoprecipitated with anti-His beads and immunoblotted with anti-HA antibody.
-
As reported previously, FBXW7 interacted with SHP2 and mediated the degradation and ubiquitination of SHP2, thus disrupting the SHP2/c-Cbl complex, which mediated RIG-I degradation and type Ⅰ interferon signaling (Song et al. 2017). As PML had been shown to increase the FBXW7 protein expression, we suspected that PML may affect SHP2 and RIG-I and inhibit the IAV replication by pro-moting the production of type Ⅰ IFN.
To determine whether PML interacted with SHP2, the Myc-PML and HA-SHP2 plasmid was transfected into HEK293T/17 cells. The total cell extracts were extracted and immunoprecipitated with anti-Myc or anti-HA anti-body followed by Western blot analysis. The result showed that PML interacted with SHP2 (Fig. 5A and 5B). Further immunoprecipitation assay showed that PML interacted with endogenous SHP2 when HEK293T/17 cells were transfected with Myc-PML plasmids and infected with IAV for 24 h (Fig. 5C). Moreover, the level of SHP2 protein expression was decreased in A549 cells transfected with Myc-PML plasmid (Fig. 5D). The cycloheximide chase assay showed that overexpression of PML synergized with FBXW7 to decrease the half-life of intracellular SHP2 protein in HEK293T/17 cells (Fig. 5E). PML interacted with FBXW7 and SHP2, thereby forming an interaction complex between PML, FBXW7, and SHP2. To investi-gate the role of PML in the interaction between FBXW7 and SHP2, HEK293T/17 cells were co-transfected with Myc-PML and His-FBXW7 plasmids together and we observed that PML enhanced the interaction between FBXW7 and SHP2 (Fig. 5F). These data demonstrated that PML enhanced the interaction between FBXW7 and SHP2 and promoted degradation of SHP2.
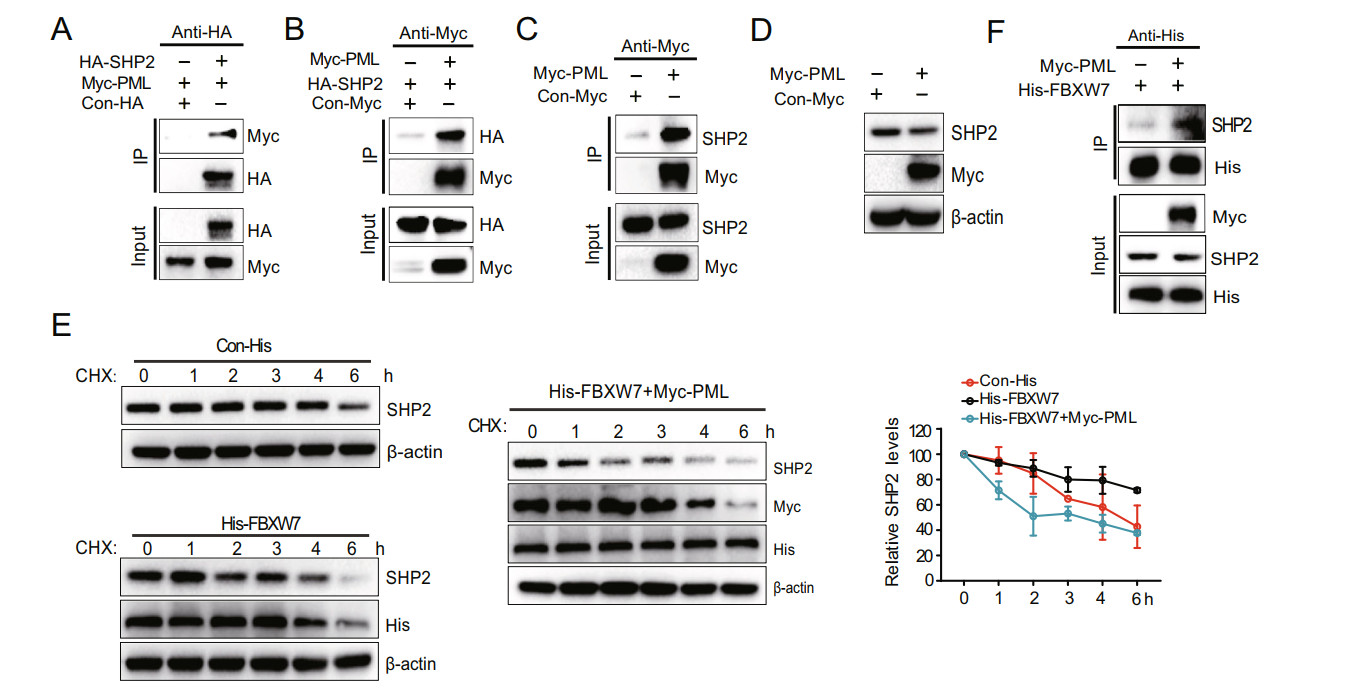
Figure 5. PML enhances the interaction between FBXW7 and SHP2 and promotes degradation of SHP2. A and B HEK293T/17 cells were co-transfected with HA-SHP2 and Myc-PML plasmids for 36 h. The cell lysates were immunoprecipitated with anti-HA or anti-Myc beads and immunoblotted with anti-Myc or HA antibody. C HEK293T/17 cells were transfected with Myc-PML plasmids for 24 h. Coimmunopre-cipitation and immunoblot analysis of HEK293T/17 cells infected with IAV at 24 hpi. D A549 cells were transfected with Myc-PML plasmid (1 μg) and Myc vector (1 μg) for 36 h. Myc-PML and SHP2 protein expression were analyzed by Western blot. E HEK293T/17 cells were transfected with Con-His, His-FBXW7, and Myc-PML for 24 h and incubated with cycloheximide (CHX, 10 μg/mL) for indicated times. The experiments were performed in triplicate. Each value represents mean ± SD. F HEK293T/17 cells were co-trans-fected with Myc-PML and His-FBXW7 for 24 h. The cell lysates were immunoprecipitated with anti-His beads and immunoblotted with SHP2 antibody.
-
Considering the previous report that FBXW7 degraded SHP2 protein while SHP2/c-Cbl complex negatively reg-ulated RIG-I, we speculated that PML may promote RIG-I protein expression (Song et al. 2017). To detect whether PML could affect the RIG-I, A549 cells were transfected with Myc-PML or si-PML with or without IAV infection. The results showed that the protein expression of RIG-I was significantly increased in both infected and non-in-fected cells with PML overexpression (Fig. 6A), while RIG-I was downregulated in PML knockdown cells (Fig. 6B). The cycloheximide chase assay showed that PML overexpression extended the half-life of endogenous RIG-I protein (Fig. 6C). These data indicate that PML regulates type Ⅰ IFN signaling by protecting RIG-I from degradation. To further confirm the role of PML, we detected IFN-α and IFN-β mRNA levels in RAW264.7 cells that were transfected with Myc-PML and infected with IAV. PML overexpression cells displayed higher IFN-α and IFN-β mRNA level compared with control cells (Fig. 6D and 6E). Overall, these data demonstrated that PML stabilized RIG-I and promoted the production of type Ⅰ IFN.
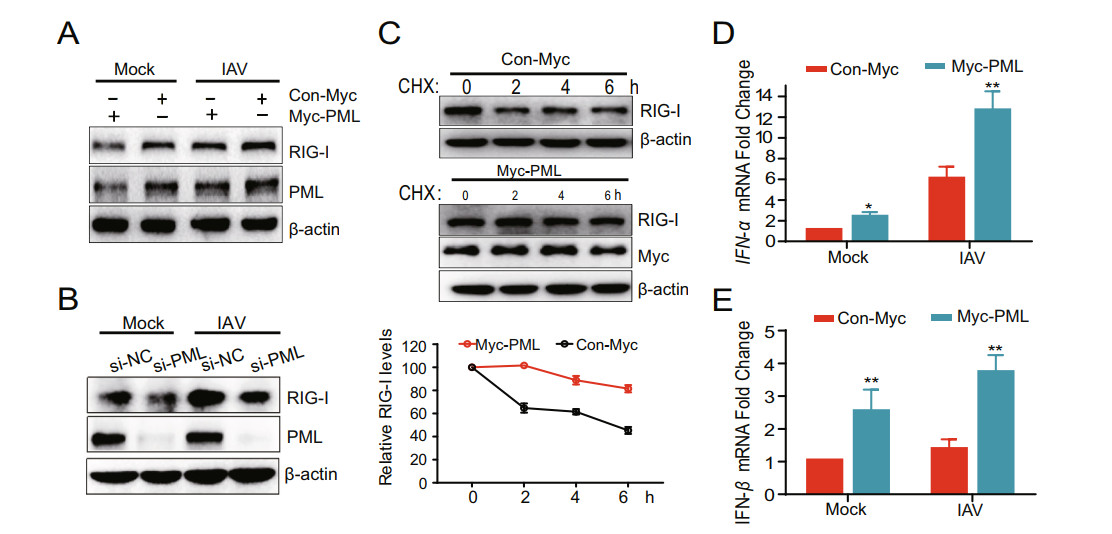
Figure 6. PML represses the degradation of RIG-I and promotes type Ⅰ IFN production. A A549 cells transfected with Myc-PML plasmid (1 μg) and Myc vector (1 μg) for 24 h were infected with IAV (MOI = 0.2) for 2 h. After 24 hpi, PML and RIG-I expressions were analyzed by Western blot. B A549 cells transfected with si-PML (20 nmol/L) and si-NC (20 nmol/L) for 24 h were infected with IAV (MOI = 0.2) for 2 h. After 24 hpi, PML and RIG-I expression were analyzed by Western blot. C A549 cells were transfected with Myc-Con or Myc-PML for 24 h and incubated with cycloheximide (CHX, 15 μg/mL) for indicated times. D and E RAW264.7 cells transfected with PML plasmid (1 μg) and vector (1 μg) for 24 h were infected with IAV (MOI = 0.2) for 2 h. The cellular IFN-α and IFN-β RNA levels were determined by qRT-PCR at 24 hpi. The experiments were performed in triplicate. Each value represents mean ± SD. *P < 0.05; **P < 0.01 versus Myc group.
IAV Infection Upregulates the Expression of PML in A549 Cells
PML Inhibits IAV Infection
PML Interacts with FBXW7
PML Represses Ubiquitylation and Degradation of FBXW7
PML/SHP2 Interaction Promotes the Degradation of SHP2
PML Stabilizes RIG-I and Promotes the Production of Type Ⅰ IFN
-
In this study, the results showed that the protein and mRNA levels of PML and the number of PML nuclear bodies significantly increased after infection with influenza virus, indicating that PML protein was positively correlated with influenza virus infection. Reports indicated that overexpression of PML significantly delayed influenza viral replication rate in Caco-2 cells, and downregulation of PML expression by RNAi enhanced viral replication (Li et al. 2009; Chelbi-Alix et al. 1998). We also found that overexpression of PML IV in A549 cells inhibited the replication of subtype A/H1N1 (Fig. 2A–2D) and subtype A/H3N2 (Fig. 2G) viruses, and knockdown PML increased the sensitivity of H1N1 and H3N2 viruses, which were consistent with the previous reports (Li et al. 2009; Iki et al. 2005). However, Li et al. showed that overexpression of PMLVI in MDCK cells conferred potent resistance to PR8 (H1N1) infection, while lacked inhibitory activity to ST1233 (H1N1), ST364 (H3N2), Qa199 (H9N2), and Ph2246 (H9N2) (Li et al. 2009). The different PML iso-forms and different subtype influenza viruses may be responsible for the discrepancy.
Mechanistically, we found that PML inhibited the virus by regulating FBXW7-SHP2-RIG-I signaling pathway. It has been reported that the effect of FBXW7 on the innate immune response to inhibit virus relied on the interferon signaling pathway. Song et al. reported that FBXW7 translocated from nucleus into the cytoplasm during virus infection, which is critical for its antiviral function (Song et al. 2017). Despite PML proteins are primarily located in the nucleus, a small portion of PML is distributed in cytoplasm (Zhong et al. 1999). Therefore, we conducted Co-IP assay on the isolated cytoplasm and nuclear proteins respectively, and found that the interaction between PML and FBXW7 was both located in the cytoplasm and nuclear. The result suggested that PML may play an antiviral role through FBXW7 mediated downstream sig-naling pathway in cytoplasm. How does PML undergo nucleocytoplasmic shuttling? As we mentioned above, the localization of the majority of PML proteins was restricted to the nucleus due to the NLS locating in exon 6 of PML. However, a fraction of PML resides in the cytoplasm (Lin et al. 2004; Giorgi et al. 2010). In addition, PML provides sites for posttranslational modifications, for example, acetylation and phosphorylation sites surrounding the NLS (Hayakawa et al. 2008; Reineke et al. 2008) and it is reasonable to assume that these modifications by FBXW7 or other factors alter the accessibility to the NLS.
Increasing evidence suggests that E3 ligases play important roles in antiviral innate signaling. Although PML is a member of RBCC/TRIM family, the E3 ubiquitin ligase activity of PML has not been reported (Janer et al. 2006; Nisole et al. 2005). In this study, we demonstrated that PML maintained the stability of FBXW7 by inhibiting the K48-linked ubiquitination and degradation of FBXW7, which suggested that the interaction between PML and FBXW7 might inhibit the other E3 ubiquitin ligase to modify the FBXW7. However, this detail is not yet clear.
Previous study had showed that FBXW7 interacted with SHP2 and mediated the degradation and ubiquitination of SHP2 (Song et al. 2017), so we next studied the effect of PML on SHP2. The results showed that PML interacted with SHP2, and the interaction of PML with FBXW7 and SHP2, respectively, further enhanced the connection between FBXW7 and SHP2, and promoted the FBXW7-mediated degradation of SHP2. Three possible reasons may explain the results. First, the close binding between FBXW7 and SHP2 facilitated the recognition of SHP2 by FBXW7, which promoted ubiquitination and degradation. Second, PML increased the expression of FBXW7 and indirectly enhanced the E3 ubiquitin ligase activity of FBXW7 and promoted the degradation of SHP2. Third, PML may degrade SHP2 through other ubiquitin–protea-some pathways, such as SUMO or NEDD, which need further study.
RIG-I recognized cytoplasmic influenza virus RNA, activated the downstream transcription factors IRF3 and IRF7, and induced type Ⅰ interferon production, thus playing the role of antiviral natural immune regulator (Kell and Gale 2015). During RNA viral infection, SHP2 as a connector protein can connect the E3 ubiquitin ligase c-Cbl to the RIG-I to mediate k48-linked ubiquitination of RIG-I (Chen et al. 2013). Our results showed that the overex-pression of PML inhibited RIG-I degradation by regulating SHP2, thus significantly increasing the mRNA level of IFN-α and IFN-β. Interestingly, the interferon can also induce the transcription and translation of PML and Sp100 in the PML-NBs, which further increased the number and volume of PML-NBs in the nucleus (Chelbi-Alix et al. 1995). Therefore, we believe that there is positive feedback regulation between PML and IFN.
In conclusion, PML upregulated the protein level of FBXW7 by inhibiting its K48-linked ubiquitination. Moreover, PML interacted with FBXW7 and SHP2 to form PML-FBXW7-SHP2 complexes, which promoted the degradation of SHP2. The reduction of SHP2 feedback maintained the stability of RIG-I and further promoted the production of type Ⅰ interferon to inhibit the replication of the influenza virus. This research partly revealed the mechanism of PML on the influenza virus. The research on these host proteins not only helped to understand the replication of the influenza virus, but also provided a potential target for the development of novel treatment to combat influenza virus infection.
-
The work was financially supported by National Science and Technology Major Projects for "Major New Drugs Innovation and Development" (2018ZX09711003), CAMS Initiative for Innovative Medicine (CAMS-I2M-1-010), and National Natural Science Foundation of China (81630089).
-
HYY, KL and YHL designed the experi-ments. HYY and MZ carried out the experiments. HYY, MZ and LY analyzed the data. HYY, HQW, and SW wrote the manuscript. KL and YHL finalized the manuscript. All authors read and approved the final manuscript.
-
No potential conflict of interest was reported by the authors.
-
This study does not contain any studies with human participants or animals performed by any of the authors.
















 DownLoad:
DownLoad: