HTML
-
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality in the children younger than five years old, especially in developing countries (DeAntonio et al. 2016; Ning et al. 2017). There are approximately 150 million children with CAP each year, with the huge financial burdens to society and family (Zhang et al. 2013; Jain et al. 2015). Early and accurate diagnosis of the aetiological cause would be helpful for effective treatment and management of children with CAP and significantly reducing mortality (Caglayan Serin et al. 2014; Chen et al. 2015). Respiratory viruses are recognized as the leading cause of children with CAP and can be detected in more than 50% of the cases (Padilla Ygreda et al. 2010; Ning et al. 2017; Oumei et al. 2018). Several studies have suggested that the role of respiratory viruses in children with CAP has been underestimated, due to the application of low sensitive detection methods (Wang et al. 2016; Chen et al. 2017; Xu et al. 2017). With the development of molecular diagnostic technologies, the novel detection assays have been increasingly applied in the diagnosis of infectious disease (Cantais et al. 2014; Mathew et al. 2015). However, the multicenter study on the profile of viral pathogens in children with CAP is scarce in Chinese mainland.
Here, we performed a multicenter study to investigate the feature of viral aetiology of hospitalized children with CAP by using multiplex PCR assay in China, and to analyze the relationship between respiratory viruses and clinical severity.
-
From November 2014 to June 2016, a prospective study was conducted in 13 children hospitals or comprehensive hospitals in north China (7 hospitals, including Beijing Children's Hospital, Capital Medical University in Beijing, Shengjing Hospital of China Medical University in Liaoning Province, Children's Hospital Capital Institute of Pediatrics in Beijing, Children's Hospital of Changchun in Jilin Province, Yinchuan Women and Children Healthcare Hospital in Ningxia Hui Autonomous Region, Baoding Children's Hospital in Hebei Province and The 2nd Affiliated Hospital of Harbin Medical University in Heilongjiang Province) and south China (7 hospitals, including the Children's Hospital of Zhejiang University School of Medicine in Zhejiang Province, Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine in Shanghai, The 2nd Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University in Zhejiang Province, Guangzhou Women and Children's Medical Center in Guangdong Province, The First Affiliated Hospital of Guangzhou Medical University in Guangdong Province, Children Hospital of Chongqing Medical University in Chongqing).
The diagnostic criteria of CAP as follows: patients can be diagnosed as CAP by meeting any of the following (1)–(4) and (5) term with the exception of tuberculosis, non-infectious interstitial pulmonary diseases, pulmonary edema and other diseases. (1) The symptoms of cough, expectoration or preexisting respiratory diseases are increased, and purulent sputum is accompanied with or without chest pain; (2) fever; (3) signs of pulmonary consolidation and/or audible and moist rales; (4) white blood cell (WBC) > 10 × 109 /L or < 4 × 109 /L, with or without nucleus left; (5) chest X-ray showed patchy, invasive, or interstitial changes with or without pleural effusion (Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association et al. 2013).
All the hospitalized children diagnosed with CAP would be screened. Children were included if their disease course were less than seven days. Cases were excluded if they were diagnosed as pneumonia after 48 h of admission.
The severe CAP case was defined by several factors, including cough or difficulty breathing plus one of the following: lower chest indrawing, nasal flaring, or grunting. Very severe pneumonia case was defined as cough or difficulty breathing plus one of the following: cyanosis, severe respiratory distress, inability to drink or vomiting everything, or lethargy/unconsciousness/convulsions (Subspecialty Group of Respiratory Diseases, The Society of Pediatrics, Chinese Medical Association et al. 2013).
-
Respiratory specimens, including throat swabs or nasopharyngeal aspirates (NPAs), were collected by trained nurses in the first 24 h of hospitalization and transported to lab in viral transport media (VTM). All specimens were stored at − 80 ℃ until use. The demographic, epidemiologic and clinical data were recorded using a uniform case report form (CRF) by clinical researchers. All the information of CRFs was uploaded to the clinical research information platform.
-
Total nucleic acids (DNA and RNA) were extracted from 200 μL throat swabs or NPAs specimens using QIAamp MinElute Virus Spin Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and eluted in 60 μL elution buffers. The presence of 18 respiratory viruses, including respiratory syncytial virus (RSV A or RSV B), parainfluenza virus (PIV) type 1–4, 2009 H1N1 influenza virus (2009 H1N1), H3 subtype influenza virus (H3), seasonal H1 subtype influenza virus (H1), influenza B virus (Flu B), human rhinovirus/enterovirus (HEV/HRV), human coronavirus (HCoV 229E, NL63, HKU1, and OC43), human metapneumovirus (HMPV), human bocavirus (HBoV), and human adenovirus (HAdV), were simultaneously screened using Xtag RVP Fast V2 kit (Luminex, USA) with Luminex MAGPIX system. An internal positive control (MS2) was added into each specimen before the nucleic acid extraction, and a positive PCR control (Lambda DNA) was added in every PCR batch run, following the manufacturer's manual. VTM only served as a negative control for nucleic acid extraction. HEV/HRV-positive samples were further performed real-time RT-PCR for further typing HEV and HRV by using universal nucleic acid detection kit for human enterovirus (Bio-Germ, Shanghai, China) and universal nucleic acid detection kit for human rhinovirus (Bio-Germ).
-
Continuous variables were summarized as means ± standard deviations (SD) or median. Categorical variables are displayed as frequencies and percentages using the chi-square test or the Fisher's exact test. The continuous variables were compared using a one-way analysis of variance (ANOVA). A P value < 0.05 was considered statistically significant. All analyses were performed using SPSS software, version 19.0 (IBM Corporation, USA).
Study Design and Enrollment of Patients
Specimen and Clinical Data Collection
Molecular Detection of Viral Pathogens
Statistical Analysis
-
A total of 3480 children who diagonsed with CAP from 13 hospitals in Chinese mainland during November 2014 to June 2016 were screened in this study. Of 3480 children, 2721 (78.2%) patients were recruited finally, and the other patients (21.8%) were exculded because of rejection of guardians (82.2%) or no respiratory specimen (17.4%) (Fig. 1). The demographic and clinical characteristics of recruited cases were list in Table 1.
Characteristics n (%) Gender Male 1698 (60.7) Female 1092 (39.3) Age Median 2.17 y Age group <6 m 413 (15.2) 6–12 m 362 (13.3) 1–3 y 824 (30.2) 3–5 y 511 (18.8) 5–18 y 611 (22.5) Symptom Fever 2043 (75.1) Cough 2664 (97.9) Expectoration 1012 (37.2) Wheeze 985 (36.2) Hemoptysis 16 (0.6) The major complications of severe pneumonia case 240 (8.8) Acute respiratory failure 77 (32.1) Pleural effusion 42 (17.5) Atelectasis 26 (10.8) Cardiac damage 9 (3.8) CAP: community-acquired pneumonia Table 1. Demographic and clinical data of children with CAP
-
Viral pathogens were detected in 56.6% (1539/2721) of the cases, with 39.8% (1082/2721) single virus infection and 16.8% (457/2721) multiple virus infection. HEV/HRV (73.1%, 334/457) was the most common virus associated with co-infection, followed by RSV (47.9%, 219/457), HMPV (30.0%, 137/457), HPIVs (26.5%, 121/457), HBoV (20.8%, 95/457) and HAdV (14.2%, 65/457). In north China, viral pathogen was detected in 54.6% (616/1128) of the cases: single virus infection in 35.5% (401/1128) of the cases and multiple virus infection in 19.1% (215/1128) of the cases. In south China, viral pathogen was detected in 57.9% (923/1593) of the cases: single virus in 42.7% (681/1593) of the cases and multiple viruses in 15.2% of the case (242/1593) (Fig. 2). There was no statistic difference in detection rate of recruited children between north China and south China (χ2 = 2.98, P = 0.0843).
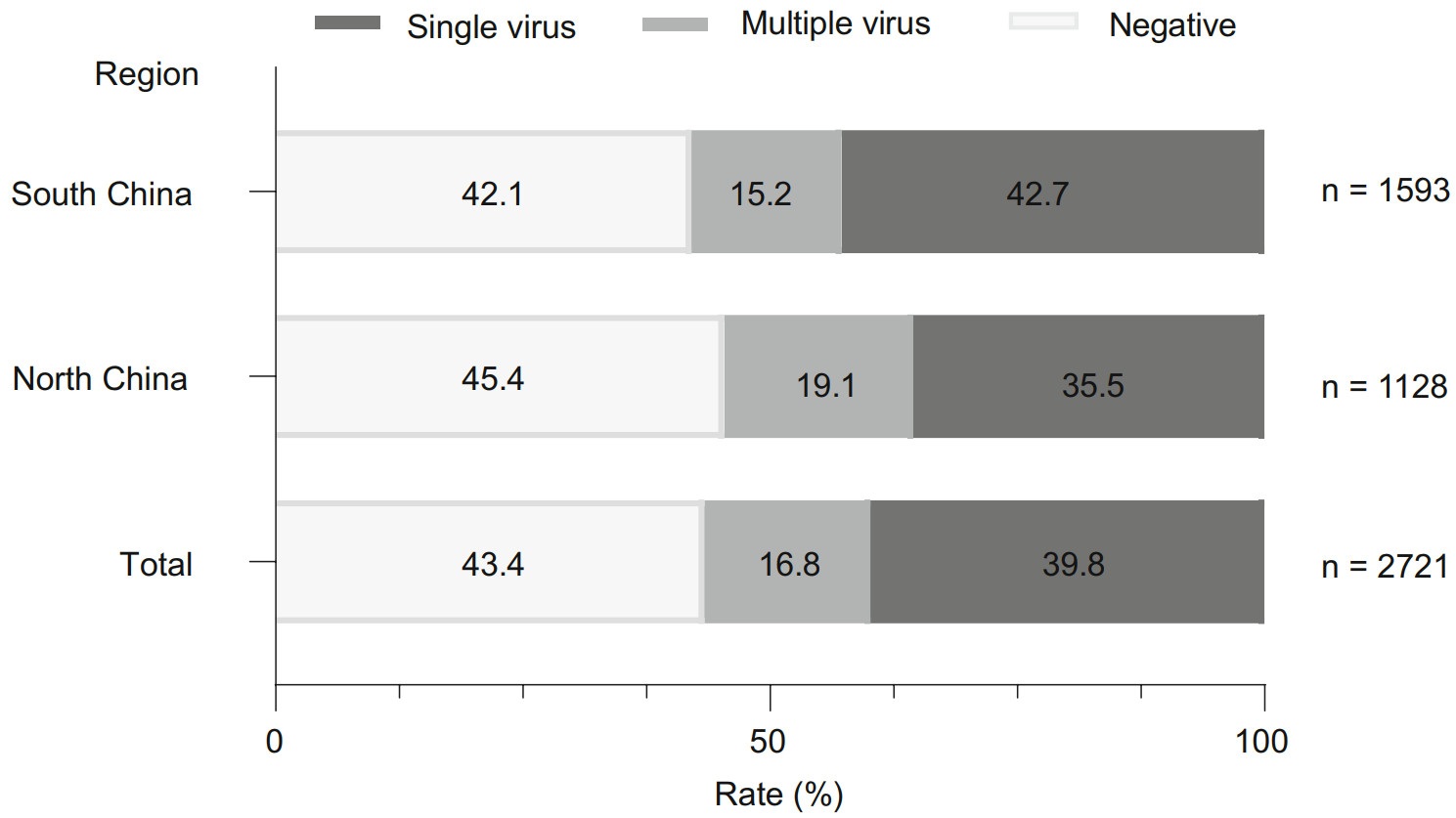
Figure 2. Detection rate of viral pathogens in children with CAP. CAP: community-acquired pneumonia.
The most frequently detected viral pathogen was RSV (15.2%, 413/2721), followed by HEV (13.4%), HRV (10.2%) [The HEV/HRV was detected in 23.6% (643/2721) of the cases by using Luminex RVP Fast V2 kit. To further classify the HEV or HRV infection, real-time RT-PCR (Taqman probe) assay were performed in 180 available HEV/HRV-positive samples. The HEV- and HRV-positive samples accounted for 56.7% (102/180) and 43.3% (78/180), respectively. According to the proportion, the detection rate of HEV and HRV should be 13.4% and 10.2%, respectively], HPIVs (9.0%, 244/2721), HBoV (8.7%, 238/2721), HMPV (7.8%, 213/2721), HAdV (5.7%, 154/2721), Flu A (2.5%, 75/2721), HCoVs (2.6%, 71/2721), Flu B (2.5%, 69/2721) (Fig. 3). For HPIVs, the detection rate of HPIV1–4 was 1.6% (44/2721), 1.4% (37/2721), 5.2% (141/2721), 1.1% (31/2721), respectively. For HCoVs, the most common detected virus was HCoV-OC43 (1.3%, 36/2721), and the positive rate of other three HCoVs, 229E, NL63, HKU1, was 0.6% (15/2721), 0.1% (4/2721), 0.7% (18/2721), respectively. In 75 Flu A-positive samples, the 2009 pandemic H1N1 influenza A virus accounted for 20% (15/75), H3 subtype influenza A virus accounted for 22.7% (17/75), while the rest part was unsubtyped Flu A (57.3%, 43/75). The detection rate of HPIVs, HBoV and Flu B in north China were significantly higher than that in south China (HPIVs: χ2 = 8.735, P = 0.0031, HBoV: χ2 = 14.99, P = 0.0001 and Flu B: χ2 = 25.28, P < 0.0001). However, the detection rate of RSV and HMPV in north China were significantly lower than that in south China (RSV: χ2 = 11.67, P = 0.0006 and HMPV: χ2 = 8.735, P = 0.0031). There were no statistic differences in detection rate of HEV/HRV, HAdV, Flu A and HCoVs between cases in north and south China.

Figure 3. Detection rate of different viruses or virus subtypes in children with CAP. CAP: community-acquired pneumonia; RSV: respiratory syncytial virus; HEV/HRV: human enterovirus/human rhinovirus; HPIVs: human parainfluenza viruses (including HPIV1–4); HBoV: human bocavirus; HMPV: human metapneumovirus; HAdV: human adenovirus; Flu A: influenza A virus (including 2009 pandemic H1N1 influenza A virus, H3 subtype influenza A virus); HCoVs: human coronavirus (including HCoV-229E, -OC43, -NL63 and -HKU1); Flu B: influenza B virus. The symbol "*" indicated that there was statistic difference in the detection rate between north and south China using the chi-square test or the Fisher's exact test. A P < 0.05 was considered statistically significant.
Severe pneumonia patients accounted for 8.8% (240/2721) in children with CAP. The major complications included acute respiratory failure (32.1%, 77/240), pleural effusion (17.5%, 42/240), atelectasis (10.8%, 26/240), cardiac damage (3.8%, 9/240) (Table 1). Of all severe pneumonia cases, 40.8% (98/240) patients were infected with single virus, while 19.2% (46/240) patients were co-infected with multiple viruses. However, 40.0% (96/240) severe pneumonia cases were viral pathogen-negative. HEV/HRV (27.6%, 27/98), HBoV (18.4%, 18/98), RSV (16.3%, 16/98) and HMPV (14.3%, 14/98) were the most common detected viral pathogens in patients with single virus infection (Fig. 4).
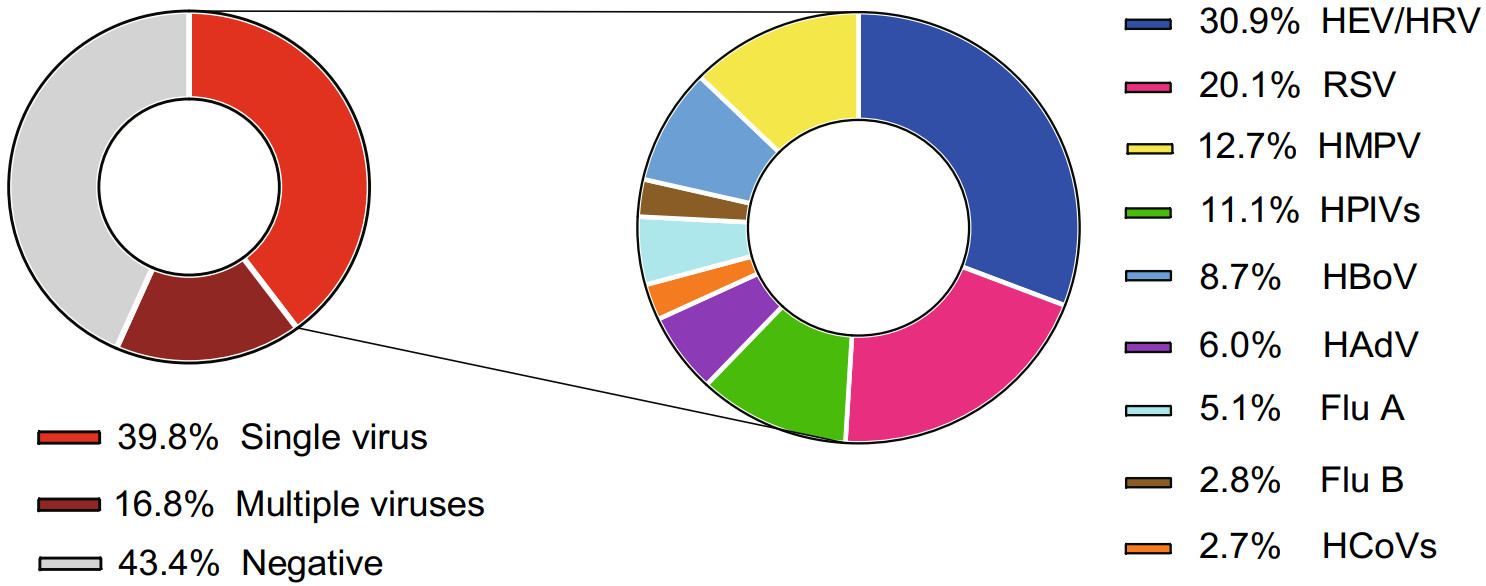
Figure 4. Spectrum of viral pathogens in severe CAP cases. CAP: community-acquired pneumonia; RSV: respiratory syncytial virus; HEV/HRV: human enterovirus/human rhinovirus; HPIVs: human parainfluenza viruses (including HPIV1–4); HBoV: human bocavirus; HMPV: human metapneumovirus, HAdV: human adenovirus; Flu A: influenza A virus (including 2009 pandemic H1N1 influenza A virus, H3 subtype influenza A virus); HCoVs: human coronavirus (including HCoV-229E, -OC43, -NL63 and -HKU1); Flu B: influenza B virus.
-
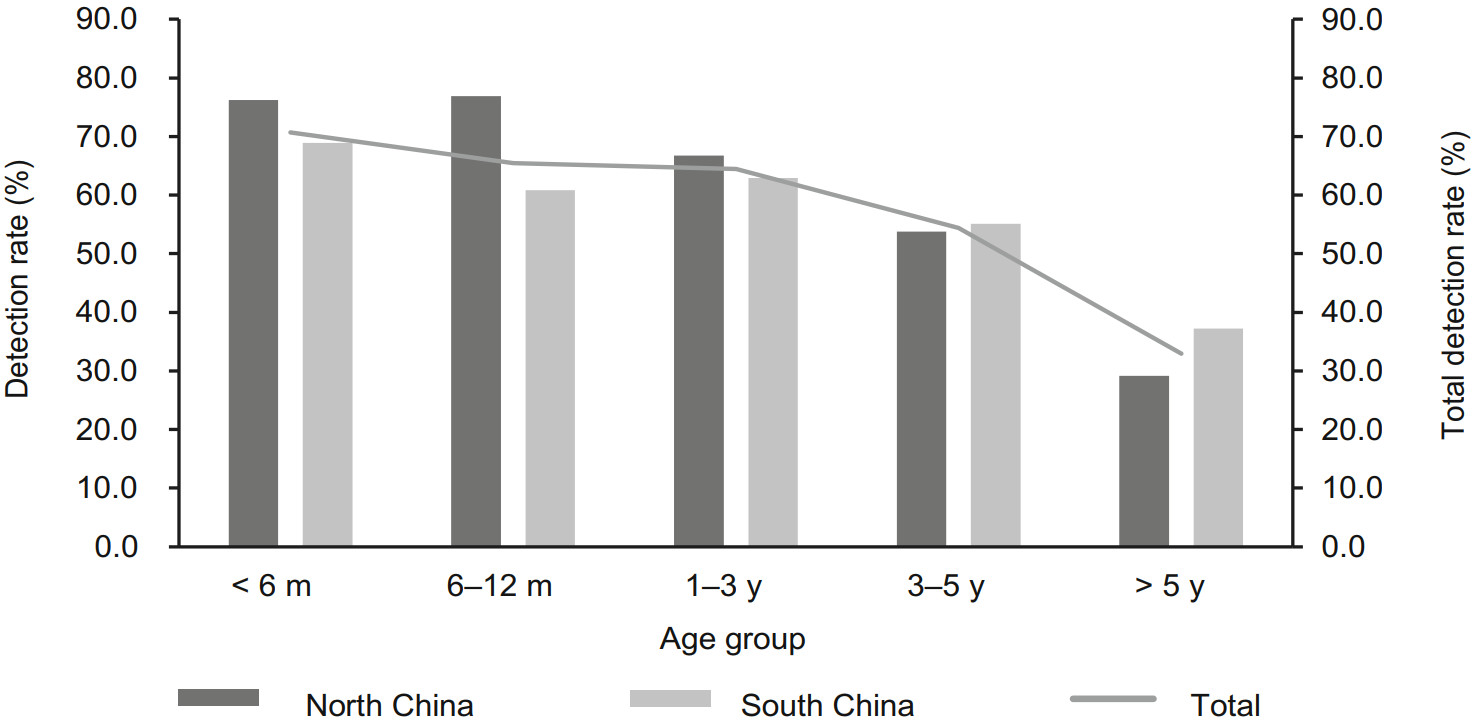
The detection rates of viral pathogen decreased gradually with the increase of age. The detection rate of virus was the highest in children younger than six months old (70.7%, 292/413). There were significantly difference among five age groups (χ2 = 207.89, P < 0.001) (Fig. 5). RSV and HRV/HEV were the most frequently detected viruses in < 6 months, 6–12 months and 1–3 years groups. HBoV, HPIVs and HAdV were more commonly detected in children aged between 1–3 years. HMPV showed more often in children younger than five years. And similar detection rates were found among < 6 months, 6–12 months, 1–3 years and 3–5 years age groups. Flu A and Flu B showed the highest detection rate in cases between 1–3 years old and 3–5 years old, respectively. HCoVs were the most common pathogens appeared in < 6 months group (Supplementary Table 1).
-
RSV, HMPV and Flu B infection peaked in winter both in north and south China. Infection of Flu A reached the peak in winter and spring in north China, while peaked in summer in south China. HPIV infection was most frequently detected in summer both in north and south China. HEV/HRV infection circulated all year round, which showed higher detection rate from spring to summer. HBoV were more prevalent in summer in north China, but the peak of HBoV infection occurred in winter and summer in south China. Though HAdV was detected more often during winter than other seasons in north China, it kept lower prevalence in south China. The detection rate of HCoVs ascended in spring and summer in north China, which showed lower level in south China all year round (Fig. 6A, 6B).
The Demographic and Clinical Features of Patients
Viral Pathogen Detection
Comparison of Detection Rate of Viral Pathogens in Different Age Groups
Seasonal Characteristic of Viral Pathogens in North and South China
-
Here we performed a prospective, multicenter study of viral aetiology of CAP in hospitalized children at 13 sites nationwide from 2014 to June 2016. In this study, we used the multiplex assay to simultaneously detect 18 respiratory viruses in respiratory specimens from children with CAP. The assay exerted higher sensitivity and specificity, and provided clinicians with more accurate diagnosis and treatment information (Choudhary et al. 2016). In this study, the overall detection rate of viral pathogen was 56.6%, which was similar in north (54.6%) and south China (57.9%). The previous studies reported that respiratory virus infection was present in 30%–73% of hospitalized children with CAP (Ning et al. 2017; Oumei et al. 2018). The various results between our study and previous reports might be attributed to the difference of study population, study region, the year of analysis or detection method.
In Chinese mainland, the incidence of pneumonia in children younger than 5 years of age ranged from 0.06–0.27 episodes per person-year and mortality varied between 184 and 1223 deaths per 10, 000 population, which caused huge social and economic burdens (Guan et al. 2010). In present study, children under 5 years old with CAP accounted for 77.5% (2110/2721) of cases. The most commonly detected viral pathogens were RSV (18.9%), followed by HEV (13.9%), HRV (11.7%), HPIVs (10.4%), HBoV (10.1%), HMPV (9.1%), HAdV (5.9%), Flu (5.1%, Flu A 2.7%; Flu B 2.4%) and HCoVs (3.0%) (The detection rate of HEV and HRV were estimated based on the results of real-time RT-PCR of 162 available specimens from children under 5 years old). A systematic review indicated that RSV (17.3%), aside from HRV (20.3%, detected in only two studies), was the most common detected viruses, followed by HBoV (9.9%), HPIVs (5.8%), HMPV (3.9%), and Flu (3.5%) in children under 5 years old with CAP in Chinese mainland (Ning et al. 2017). Because HRV was detected only in two studies with small sample size, the detection rate of HRV in this review might be a statistical bias. Another study reported that the most frequently identified viruses were RSV (28%), HRV (27%), HMPV (13%), HAdV and Flu (7%) and HCoVs (5%) among U.S. hospitalized children with CAP (Jain et al. 2015). Together, these studies suggested that RSV was the most frequently detected viral pathogen in children with CAP.
RSV is the most important viral agent of CAP in children, which is also the most frequently detected viral pathogen in children with CAP under 5 years old. The detection rate of RSV infection declined gradually with increasing of age (Berkley et al. 2010; Nair et al. 2010). In this study, the detection rate of RSV in patients less than 5 years old was the highest (18.9%). Another multicenter study using direct immunofluorescence assays (DFA), reported that RSV was the most predominant viral pathogen (11.5%) in 1500 hospitalized children with CAP in Chinese mainland (Oumei et al. 2018), which was similar to our result, but lower than ours, due to the poor sensitivity of DFA assay.
HEV and HRV were members of the Picornaviridae family, which was underestimated its important role in CAP before (Garcia et al. 2013; Ren et al. 2017). HRV infections in young children were usually subclinical or accompanied by other respiratory pathogens. However, HRV and HEV were both thought to be possessed the capacity for viremia (Lu et al. 2017). Another study reported that HRV (21%) and HEV (9%) were the second and third etiological cause of bronchiolitis in hospitalized infants after RSV in French (Jacques et al. 2006). A 2-year prospective study reported that RSV (27%), HEV (25%) and HRV (24%) were the most important causative agents of acute expiratory wheezing in children in Finland (Jartti et al. 2004). These results were consistent with our study, which highlighted that more attention was needed for the important role of HEV and HRV in children with CAP.
HPIVs infection accounted for a significant proportion of lower respiratory tract infections in children (Ljubin-Sternak et al. 2016). In another study that multiplex real-time PCR assay was used, HPIVs were identified in 9.07% of the cases with severe acute respiratory infection in Beijing, China (Pan et al. 2017). In this study, HPIVs was the fourth most common causative agent, and four types of HPIVs were all detected. HPIV3 was the most predominant type, which was consistent with other studies (Thomazelli et al. 2017).
HBoV was identified in 2005 by Allander et al. (Allander et al. 2005). Several studies revealed the detection rate of HBoV ranged from 1.5%–24.6% (Schlaberg et al. 2017). In this study, HBoV (8.7%) was the fifth frequently detected viral pathogen, which was similar to other reports.
HMPV has been recognized as one of the most common causes of respiratory tract infection, with similar clinical manifestation to that of RSV (Papenburg and Boivin 2010). A cohort study conducted by U.S. group reported that HMPV was detected in 6% (200/3490) of the hospitalized children (Edwards et al. 2013), which was similar to, but slightly lower than that of this study.
The seasonal pattern of respiratory virus varies in viruses. In this study, HEV/HRV infection was circulating all years round, and reached peak in spring and summer both in north and south China, which was consistent to other reports (Wang et al. 2010; Jain et al. 2015). The highest detection rates of HPIVs occurred in summer both in north and south China, which were similar to our previous study on children with acute respiratory tract infection from 2010 to 2012 in Beijing (Liu et al. 2013). The peak seasons of RSV, HMPV and Flu B were winter and spring both in north or south China in 2015, which was in agreement with most previous reports (Choi et al. 2006; Pilger et al. 2011; Xie et al. 2011; Xiao et al. 2012; Lu et al. 2013). Climate factors were recognized as the strongest predictors for influenza seasonality, including minimum temperature, hours of sunshine, and maximum rainfall (Azziz Baumgartner et al. 2012; Tamerius et al. 2013). Yu and his colleagues reported that Flu A presented distinct peak activity in northern provinces, southern provinces and provinces in intermediate latitudes in China (Yu et al. 2013). Our findings supported the role of climatic factors in influenza transmission dynamics.
In recently years, the multiplex assays were increasingly applied to the diagnosis of infectious disease, with clinical significance, such as the improvement of diagnosis of co-infected cases, especially in severe pneumonia cases. In our study, multiple viral pathogens were detected in 19.1% of cases. Another study conducted among hospitalized children with CAP in U.S. showed similar results to, but higher than ours. This difference might be attributed to the difference in the populations studied, the year of analysis, and the screened pathogens (Liu et al. 2015).
In this study, viral pathogen presented in 60.0% of severe pneumonia patients. HEV/HRV, RSV, HBoV and HMPV accounted for more than 70% proportion of severe pneumonia cases infected with single virus. Our finding was consistent with the potentially severe outcome of RSV infections in children (Shi et al. 2015). However, HRV, HBoV, and HMPV usually appeared in asymptomatic cases (Debiaggi et al. 2012; Principi and Esposito 2014; Jain et al. 2015; Ren et al. 2017). Several studies revealed that the prolonged viral shedding could cause viral nucleic acid of HRV, HBoV and HMPV presented in asymptomatic patients (Jartti et al. 2008; Martin et al. 2010; Dabaniyasti et al. 2020). Therefore, further studies are needed to confirm the correlation between these virus infections and the severity of pneumonia, and interpret these controversial consequences. In addition, viral pathogens were not detected in 40% of cases in our study, probably due to bacterial, mycoplasma or other unknown virus infection.
This study has several limitations. First, only hospitalized cases with CAP were enrolled. The spectrum of viruses in outpatient children might display different results. Therefore, our findings may not be representative of entire Chinese pediatric population. Further, although this study is a multicenter study, our results may not be representative of other regions in Chinese mainland, especially in Tibet, Xinjiang, Hainan and other remote provinces. Besides, in this study, we just screened the presence of viral pathogens in respiratory specimens from children with CAP. To better understand the profile of aetiology of CAP, further study is needed to investigate the role of other causal agents, including the bacterial and mycoplasma pneumoniae in children with CAP.
In conclusion, viral pathogens are frequently found in pediatric CAP cases, which may therefore play a vital role in the aetiology of CAP in Chinese mainland. RSV is the most common virus in hospitalized children with CAP. Flu A, HBoV, HAdV and HCoVs exert distinct seasonal patterns in north and south China. More confirmed evidence is needed to elucidate the causal association between infection of HRV, HEV, HMPV and HBoV and severe pneumonia cases.
-
This study was supported by National Science and Technology Supported Projects (grant number: 2013BAI09B11), the National Major Science & Technology Project for Control and Prevention of Major Infectious Diseases in China (grant number: 2018ZX10201002-008-008, 2017ZX10103004-004). We would like to thank all participating members of 13 hospitals for their assistance and collaboration in the samples and clinical data collection.
-
KS, ZX, BX, CL, ZC, LC, ZF, YS, AC, LD, and YB conceived and designed the experiments. BX, CL, ZC, LC, ZF, YS, AC, LD, YB, YS, LN, SY, FG, JY coordinated sample acquisition and recruited the CAP patients. YZ and CL performed the experiments and analyzed the data. YZ, CL and AS wrote and/or edited the manuscript. KS, ZX and YZ revised the manuscript. All authors meet the authorship criteria and approved publication of the manuscript.
-
The authors declare that they have no conflict of interest.
-
The study protocol was approved by the Ethical Review Committee of Capital medical university, Beijing Children's Hospital (2014–99). Individual written informed consent was obtained from the parents and/or guardians of all participants.













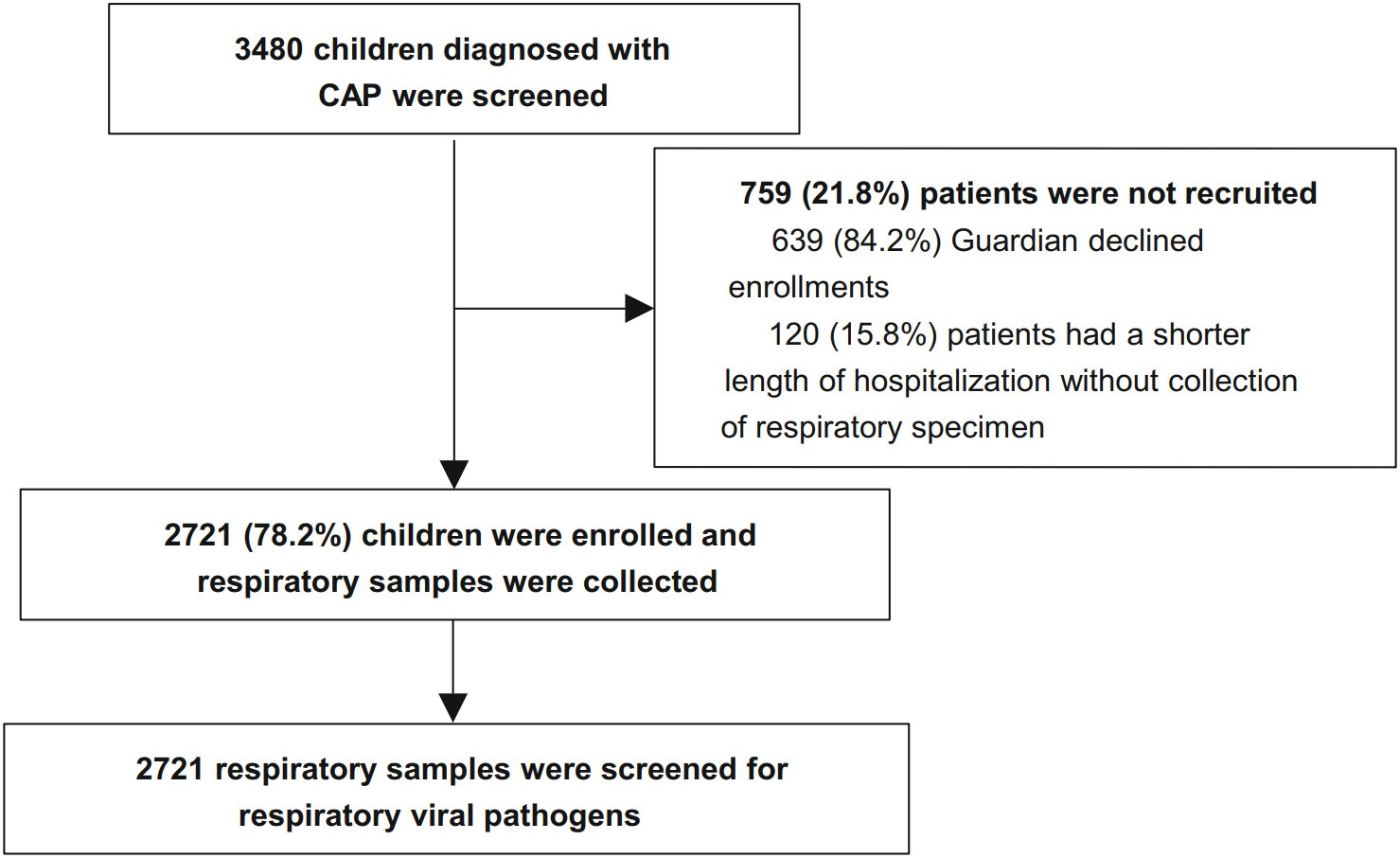




 DownLoad:
DownLoad: