-
Kaposi's sarcoma-associated herpesvirus, also called human herpesvirus 8 was first found in 1994 by Chang et al from one AIDS-KS (Kaposi's sarcoma) patient by representative differentiation assay (3). Since then, the causal link between KSHV and KS has been well established. KSHV is consistently found in all forms of KS: (classic, endemic, AIDS associated and transplantation associated). KSHV has also been observed to be associated with primary effusion B cell lymphoma (2) and multicentric Castleman's disease (10). KSHV is a large double stranded DNA virus with a size of about 210 kb; it encodes approximately 90 open reading frames (orf). To date scientists around the world have performed a number of investigations concerning the distribution and infection of KSHV in different regions. KSHV is found to have a worldwide occurrence but infection rates vary according to a combination of geographic and behavioral risk factors (10). Seroprevalence of KSHV is shown to be higher than 25% in African countries, whereas in the United States, Asia, and Western Europe is lower than 10% (1, 6, 13). Many different methods have been set up for detecting KSHV, mainly including serologic and molecular diagnosis (4, 5, 8, 11). 4 different serologic assays have been used to detect the HHV-8 antibodies; immunofluorescent assays (IFA), enzyme-linked immunosorbent assays (ELISA), Western blot and immunohistochemistry (IHC). The antigen used for ELISA can be recombinant antigen, the whole virus or the synthetic peptide but quality is important and purified antigen of high quality should be used. However, the ELISA method is simple and so it is the optimal choice for screening a large amount of sera for epidemiological research.
KSHV orf65 encodes for a small capsid protein and the recombinant orf65 has very good antigenicity and specificity, and so it represents a good choice for in the KSHV epidemiological research (7). In this study, we have cloned the c-terminal of orf65, and expressed it as recombinant protein in E.coli, and our data indicates that it possesses very good antigenicity and specificity.
HTML
-
Sal I and BamH I restriction endonuclease were purchased from Takara Co, pfu DNA polymerase and T4 DNA ligase were purchased from Promega Co, goat-anti human IgG-AP was purchased from DAKO Co.
-
TA cloning vector pGM-T was purchase from Qiagen Co, Prokaryotic expression vector pQE-80L was purchase from QIAGEN Co, Competent cells TOP10 were purchased from Invitrogen Co.
-
Primers were designed based on the nucleic acid sequence of KSHV (GenBank accession number: NC 003409), P1: 5'-ATAGGATCCGCTGACCGAGTTT CCGCGGCG-3' and P2: 5'-AGCGTCGACCGGTT GTCCAATCGTTGCCTA-3' were designed for amplifying the c-terminal of KSHV small capsid protein.
-
The genomic DNA was extracted from the KSHV positive cell line BCBL-1. The truncated orf65 was amplified with Primers P1 and P2. PCR was performed over 30 cycles of 94℃ 1 min, 57℃ 1 min and 72℃ 1 min, followed by an extension at 72℃ for 10 min. PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
-
PCR product was inserted into pGM-T vector and then transformed into TOP10 competent cells. The recombinant plasmid harboring the correct length and the direction of the truncated orf65 gene were identified by Sal I and BamH I digestion and confirmed by sequencing. The truncated orf65 gene in the pGM-T-orf65 plasmid was removed by Sal I and BamH I digestion and subcloned into pQE-80L, the recombinant plasmid was named as pQE-80L-orf65.
-
The pQE-80L-orf65 was transformed into BL21 (DE3) competent cells. The pQE-80L-orf65/BL21 (DE3) which was confirmed by PCR screening and double restriction digestion was inoculated into 5 mL LB medium containing 50μg /mL ampicillin, incubated at 37℃ until OD600 was about 0.6, then IPTG was added to the medium with the final concentration 1mmol/L. The culture was further incubated at 37℃ for another 6 h; the culture was then harvested by 5000 r/min centrifugation.
-
The harvested culture was resuspended by 10× volume PBS, ultrasonicated (300w, 3×5 sec burst) for 5 min. The culture was then added with 20% Triton X-100 to a final concentration of 1%, stirred for 30 min at room temperature and centrifuged at 10 000 r/min for 15 min. The supernatant and the precipitate was collected separately, mixed with SDS-PAGE Gel loading buffer, boiled for 10 minutes, then loaded onto 10% SDS-PAGE and electrophoresed for 1 h at 200 V.
-
The pQE-80L-orf65 was inoculated into 500 mL LB medium containing 50μg/mL ampicillin, and then 1 mmol/L IPTG was added to a final OD600 of 0.6. The culture was further incubated for another 6 h before it was harvested by centrifugation. The recombinant orf65 was then purified with Ni-NTA Agarose according to the manufacture's instructions (Qiagen Inc)
-
Proteins of the whole cell lysate and the purified recombinant orf65 were separated by SDS-PAGE and transferred by electroblotting onto a polyvinyllidene difluoride membrane. The membrane was washed and blocked with the blocking solution (PBS-T) containing 0.2% Tween 20 and incubated with KSHV positive or negative serum as the primary antibody. The membrane was washed in PBS-T (containing 0.2% Tween 20) and incubated at room temperature for 1 h with anti-human IgG-AP antibody. The membrane was washed again and visualized by adding the substrate NBT/BCIP.
-
ELISA plates were coated with 50μL of purified orf 65 (from 0.2μg /mL to 10μg /mL) in 0.1 mol/L NaHCO3 at pH 9.6 and left overnight at 4℃. A conventional ELISA protocol was used with phosphate-buffered saline containing 0.05% Tween 20 (PBS-T, PH 7.4) for washes, 5% dried skimmed milk in PBS-T (blocking buffer) to saturate plates and to dilute patient sera (from 1:100 to 1:1000), and an alkaline phosphatase conjugated affinity purified goat antihuman IgG, diluted 1 / 1 000 in blocking buffer, followed by 1 mg/mL of nitrophenyl phosphate in glycine buffer (0.1 mol/L glycine, 1 mol/L MgCl2, 1 mmol/L ZnCl, pH10.4) as substrate. The colorimetric reaction was stopped after 25 minutes at 37℃ with 100μL 3 mol/L NaOH and read spectrophotometrically at A405.
-
Purified (His) 6-tagged fusion protein orf65 was used as antigen to immunize rabbits. Rabbits were induced for 1 week following injection of 10 mg Freund's complete adjuvant (Sigma) and then immunized four times (at 2-week intervals) with the mixed purified fusion protein and Freund's incomplete adjuvant (50 mg each). Antiserum was collected at 15 days after the last immunization. Antiserum titers were determined by ELISA.
Reagents
Plasmids and bacteria
Primers design
orf65 gene amplification by PCR
Construction of prokaryotic expression plasmid pQE-80L-orf65
Expression of orf65
SDS-PAGE analysis of the expression products
Purification of recombinant orf65
Western blot analysis
Enzyme-linked immunosorbent assays (ELISA)
Preparation of orf65 antibody
-
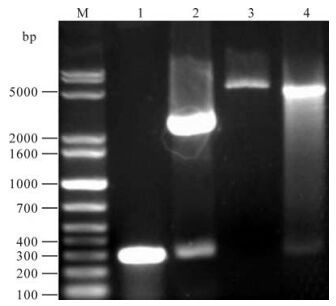
A 284 bp fragment was obtained by PCR. This fragment was cloned into pGMT-easy vector by T/A method, and the inserted sequence was identified by restriction endonuclease cleavage and sequence analysis. After digestion with Sal I and BamH I. The orf65 gene fragment was then subcloned into the pQE-80L vector by digestion with Sal I and BamH I and the recombinant plasmid was named as pQE-80L-orf65 (Fig. 1).
-
After being induced for 6 h and identified by SDS-PAGE electrophoresis, proteins of ~9 kDa were expressed in BL21 (DE3) strain transformed by pQE-80L-orf65; this is consistent with the expected expression products. Further separation of supernatant and precipitation indicated that the concentration was very high in the supernatant of the lysed BL21 (DE3) transformed by pQE-80L-orf65. High purity recombinant orf65 protein was attained after Ni2+-nitrolotriacetic acid resin affinity chromatography purification, analysis using bandscan software indicated the purity of the recombinant orf65 protein was about 99% (Fig. 2).
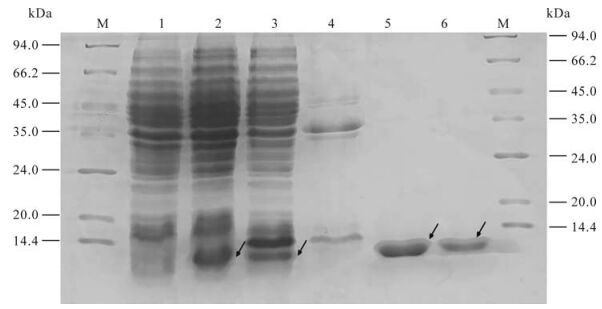
Figure 2. Expression and purification of KSHV orf65 protein. 1. The cell lysate of pQE-80l-orf65/BL21 (DE3) before IPTG induction; 2. The cell lysate of pQE-80l-orf65/BL21 (DE3) after IPTG induction; 3. The supernatant of the lysed pQE-80L-orf65/BL21 (DE3) after IPTG induction; 4. The precipitate of the lysed pQE-80L-orf65/BL21 (DE3) after IPTG induction; 5/6, Purified orf65; M, protein Marker.
-
The total protein of lysed BL21 (DE3) transformed by pQE-80L-orf65 and the purified recombinant orf65 were electrophoresed by SDS-PAGE and transferred to a PVDF membrane. The membrane was coated with the KSHV positive serum or negative serum. A specific 9 kDa band occurred in the lanes of the total and purified protein of lysed BL21 (DE3) transformed by pQE-80L-orf65 when proteins were incubated with KSHV positive serum; no band was seen when proteins were incubated with negative serum (Fig. 3). The results indicate that the recombinant orf65 has very strong specificity and antigenicity.

Figure 3. Western blot of recombinant orf65 with KSHV positive and negative serum. 1, The cell lysate of pQE-80l-orf65/BL21 (DE3) before IPTG induction; 2, The cell lysate of pQE-80l-orf65/BL21 (DE3) after IPTG induction; 3, Purified orf65; 4, The cell lysate of pQE-80l-orf65/BL21 (DE3) before IPTG induction; 5, The cell lysate of pQE-80l-orf65/BL21 (DE3) after IPTG induction; 6, Purified orf65. 1-3 reacted with KSHV positive serum; 4-6 reacted with KSHV negative serum.
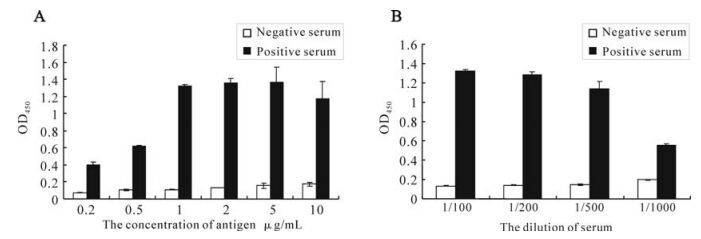
The antigenicity of the recombinant orf65 was also assessed by ELISA. The recombinant orf65 was used as antigen to coat the ELISA plates and KSHV positive and negative serum were used as the primary antibody. The ELISA result showed that the optimal antigen concentration was 1μg /mL; the optimal dilution of the serum was 1:500 (Fig. 4). The experiment was repeated three times. The result confirmed the antigenecity and specificity of the recombinant orf65 as diagnostic antigen for KSHV.
-
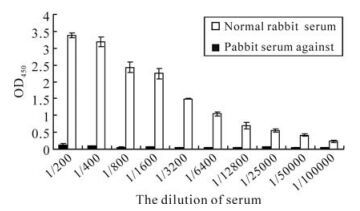
The serum titer against orf65 was detected by ELISA. The recombinant orf65 was used as antigen to coat the ELISA plates, the polyclonal anti-rabbit orf65 antibody were used as the primary antibody; the serum from the non-immunized rabbit was used as negative control. The result showed that when the rabbit serum against orf65 was diluted to 1:6 400, the OD405 value was still 23 fold compared to that of the negative control, when diluted to 1:100 000, the OD405 value was 4.9 fold compared to that of the negative control (Fig. 5). These results reveal that the polyclonal orf65 antibody could specifically identify the orf65 protein in the KSHV positive serum, and also further confirmed the strong antigenicity of the recombinant orf65.
Construction of prokaryotic expression plasmid pQE-80L-orf65
Expression and purification of recombinant orf65 protein
Identification of the antigenicity of recombinant orf65
Detection of the polyclonal orf65 antibody
-
KSHV is a DNA virus with a genome about 210 kb long. All the known open reading frames are located on a continuous 140.5 kb long unique region flanked by the 20-30kb terminal repeat region (9). Approximately 90 open reading frames (ORF) have been identified, and over 60 show homology with other rhadinoviruses. The genome also contains 15 genes which are unique to KSHV, named K1 to K15 (12).
KSHV exhibits a certain infectivity ratio among the normal healthy people who are mainly latently infected. The number of HIV-1 infection in China is rapidly increasing, and infection of HIV-1 could contribute to the pathogenesis of AIDS-associated Kaposi's Sarcoma (14). Thus, it is very important to gain greater understanding of the epidemiology of KSHV.
The orf65 gene of KSHV encodes the small virion capsid protein and is homologous to structural components of several other herpesviruses including VP26 of herpes simplex virus (HSV), BFRF3 of EBV, orf65 of HVS and the smallest capsid protein (SCP) of human cytomegaloviruses. Despite this homology, KSHV orf65 does not appear to be antigenically cross-reactive. It has been shown that antibodies to recombinant orf65 are present in nearly all patients with KS but less common in other population groups including HIV-infected patients without KS (7). So the recombinant orf65 can be used to examine the seroepidemiology of infection with KSHV in the general population.
We have cloned the c-terminal fragment encoding 100 amino acids from KSHV orf65 into the the prokaryotic expression vector pQE-80L to obtain expression plasmid pQE-80L-orf65. Recombinant orf65 could be highly expressed in the E.coli strain BL21 (DE3); the amount of the orf65 protein accounts for 24.04% of the total lysed protein. After Ni2+-nitrolotriacetic acid resin affinity chromatography purification, the purity of the recombinant orf65 could reach over 99% of the total protein.
We then used Western blot to check the antigenicity and specificity of the expressed recombinant orf65. As shown in Fig. 3, the 9 kDa bands only appeared when the orf65 proteins reacted with KSHV positive serum, but not with KSHV negative serum, and also there were no 9 kDa bands present when the total lysate from BL21 (DE3) was transformed with control vector reacted with either KSHV positive or negative serum. ELISA was also performed to check the antigenicity and specificity of the expressed recombinant orf65. As shown in Fig. 4, when the orf65 was coated at a concentration of 0.2 μg /mL, the OD405 could reach 0.4, while the negative control was only 0.06, indicating the strong antigneicity of orf65. When different concentrations of the orf65 was used to coat the ELISA plates, the OD405 values had no significant differences. Thus, 1μg /mL was chosen as the optimal concentration to use in the orf65 ELISA. Different dilutions of serum were also tried, there were not significant differences among dilutions of 1:100, 1:200 and 1:500. The serum dilution of 1:500 was then used in the orf65 ELISA.
The antigenicity of orf65 was also verified by producing the polyclonal antibody in rabbit. Orf65 was used to coat ELISA plates and then the polyclonal antibody produced in the immunized rabbit with the purified orf65 was used as the primary antibody. Our results showed that the polyclonal antibody against orf65 in the rabbit was successfully produced, and the recombinant orf65 we expressed was highly immunogenic.
The successful expression of orf65 in prokaryotic cells and the preparation of orf65 specific antibody in this report will help to establish a KSHV diagnostic method which will facilitate KSHV epidemiological research and give insight into the prevention and control of KSHV infections in China.














 DownLoad:
DownLoad: