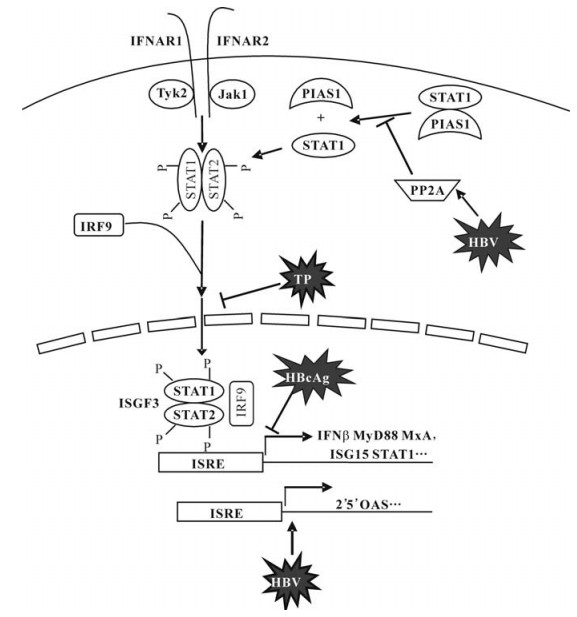
-
Bouchard M J, Schneider R J. 2004. The enigmatic X gene of hepatitis B virus.J Virol, 78: 12725-12734.
doi: 10.1128/JVI.78.23.12725-12734.2004
-
Christen V, Duong F, Bernsmeier C, et al. 2007. Inhibition of alpha interferon signaling by hepatitis B virus. J Virol, 81 (1): 159-165.
doi: 10.1128/JVI.01292-06
-
Dienes H P, Hess G, Wöorsdörfer M, et al. 1991. Ultrastructural localization of interferon-producing cells in the livers of patients with chronic hepatitis B. Hepatology, 13 (2): 321-326.
-
Duong F H, Christen V, Berke J M, et al. 2005. Upregulation of protein phosphatase 2Ac by hepatitis C virus modulates NS3 helicase activity through inhibition of protein arginine methyltransferase 1. J Virol, 79 (24): 15342-15350.
doi: 10.1128/JVI.79.24.15342-15350.2005
-
Duong F H, Filipowicz M, Tripodi M, et al. 2004. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroen-terology, 126 (1): 263-277.
doi: 10.1053/j.gastro.2003.10.076
-
Fang J W, Wu P C, Lai C L, et al. 1994. Hepatic expression of interferon-alpha in chronic hepatitis B virus infection. Dig Dis Sci, 39 (9): 2014-2021.
doi: 10.1007/BF02088140
-
Fernández M, Quiroga J A, Carreño V. 2003. Hepatitis B virus downregulates the human interferon-inducible MxA promoter through direct interaction of precore/core proteins. J Gen Virol, 84 (Pt 8): 2073-2082.
-
Foster G R, Ackrill A M, Goldin R D, et al. 1991. Expression of the terminal protein region of hepatitis B virus inhibits cellular responses to interferons alpha and gamma and double-stranded RNA. Proc Natl Acad Sci USA, 88 (7): 2888-2892. Erratum in: Proc Natl Acad Sci USA, 92 (8): 3632.
-
Foster G R, Goldin R D, Hay A, et al. 1993. Expression of the terminal protein of hepatitis B virus is associated with failure to respond to interferon therapy. Hepatology, 17 (5): 757-762.
doi: 10.1002/(ISSN)1527-3350
-
Ganem D, Pollack J R, Tavis J. 1994. Hepatitis B virus reverse transcriptase and its many roles in hepadnaviral genomic replication. Infect Agents Dis, 3: 85-93.
-
Gordien E, Rosmorduc O, Peltekian C, et al. 2001. Inhibition of hepatitis B virus replication by the interferon-inducible MxA protein. J Virol, 75 (6): 2684-2691.
doi: 10.1128/JVI.75.6.2684-2691.2001
-
Guan S H, Lu M, Grünewald P, et al. 2007. Interferon-alpha response in chronic hepatitis B-transfected HepG2.2.15 cells is partially restored by lamivudine treatment. World J Gastroenterol, 13 (2): 228-235.
-
Guo Y, Guo H, Zhang L, et al. 2005. Genomic analysis of anti-hepatitis B virus (HBV) activity by small interfering RNA and lamivudine in stable HBV-producing cells. J Virol, 79 (22): 14392-14403.
doi: 10.1128/JVI.79.22.14392-14403.2005
-
Haller O, Stertz S, Kochs G. 2007. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect, 9: 1636-1643.
doi: 10.1016/j.micinf.2007.09.010
-
Janssen H L, van Zonneveld M, Senturk H, et al. 2005. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet, 365: 123-129.
doi: 10.1016/S0140-6736(05)17701-0
-
Jilbert A R, Burrell C J, Gowans E J, et al. 1986. Cellular localization of alpha-interferon in hepatitis B virus-infected liver tissue. Hepatology, 6 (5): 957-961.
doi: 10.1002/(ISSN)1527-3350
-
Lau G K, Piratvisuth T, Luo K X, et al. 2005. Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med, 352: 2682-2695.
-
Locarnini S, Shaw T, Dean J, et al. 2005. Cellular response to conditional expression of the hepatitis B virus precore and core proteins in cultured hepatoma (Huh-7) cells. J Clin Virol, 32 (2): 113-121.
doi: 10.1016/j.jcv.2004.10.002
-
Nouri-Aria K T, Arnold J, Davison F, et al. 1991. Hepatic interferon-alpha gene transcripts and products in liver specimens from acute and chronic hepatitis B virus infection. Hepatology, 13 (6): 1029-1034. Erratum in: Hepatology, 14 (6): 1308.
doi: 10.1002/(ISSN)1527-3350
-
Nakanishi F, Ohkawa K, Ishida H, et al. 2005. Alteration in gene expression profile by full-length hepatitis B virus genome. Intervirology, 48 (2-3): 77-83.
doi: 10.1159/000081732
-
Rang A, Bruns M, Heise T, Will H. 2002. Antiviral activity of interferon-alpha against hepatitis B virus can be studied in non-hepatic cells and Is independent of MxA. J Biol Chem, 277 (10): 7645-7647.
doi: 10.1074/jbc.C100729200
-
Rosmorduc O, Sirma H, Soussan P, et al. 1999. Inhibition of interferon-inducible MxA protein expression by hepatitis B virus capsid protein. J Gen Virol, 80 (Pt 5): 1253-1262.
-
Twu J S, Lee C H, Lin P M, et al. 1988. Hepatitis B virus suppresses expression of human beta-interferon. Proc Natl Acad Sci USA, 85 (1): 252-256.
doi: 10.1073/pnas.85.1.252
-
Twu J S, Schloemer R H. 1989. Transcription of the human beta interferon gene is inhibited by hepatitis B virus. J Virol, 63 (7): 3065-3071.
-
Wang G H, Seeger C. 1992. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell, 71 (4): 663-670.
doi: 10.1016/0092-8674(92)90599-8
-
Wang G H, Seeger C. 1993. Novel mechanism for reverse transcription in hepatitis B viruses. J Virol, 67 (11): 6507-6512.
-
Wang X, Yuan Z H, Zheng L J, et al. 2004. Gene expression profiles in an hepatitis B virus transfected hepatoblastoma cell line and differentially regulated gene expression by interferon-alpha. World J Gastroenterol, 10 (12): 1740-1745.
doi: 10.3748/wjg.v10.i12.1740
-
Whitten T M, Quets A T, Schloemer R H. 1991. Identification of the hepatitis B virus factor that inhibits expression of the beta interferon gene. J Virol, 65 (9): 4699-4704.
-
Wu M, Xu Y, Lin S, et al. 2007. Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J Gen Virol, 88 (Pt 12): 3260-3269.
-
Yang J, Bo X C, Yao J, et al. 2005. Differentially expressed cellular genes following HBV: potential targets of anti-HBV drugs? J Viral Hepat, 12 (4): 357-363.
doi: 10.1111/jvh.2005.12.issue-4