HTML
-
Hepatitis C virus (HCV) is a member of the Flavi-viridae family and causes acute and chronic hepatitis. A persistent HCV infection may result in severe liver damage including liver cirrhosis and hepatocellular carcinoma (17). HCV infects only humans and chimpanzees (33). The liver is the primary target organ of HCV, and the hepatocyte is its primary target cell. Attachment of the virus to the cell surface followed by viral entry is the first step in a cascade of interactions between the virus and the target cell that is required for successful entry into the cell and initiation of infection (Fig. 1) (35). This step is an important determinant of tissue tropism and patho-genesis; it thus represents a major target for antiviral host cell responses, such as antibody-mediated virus neutralization, and antiviral therapy (for review see also (56)).
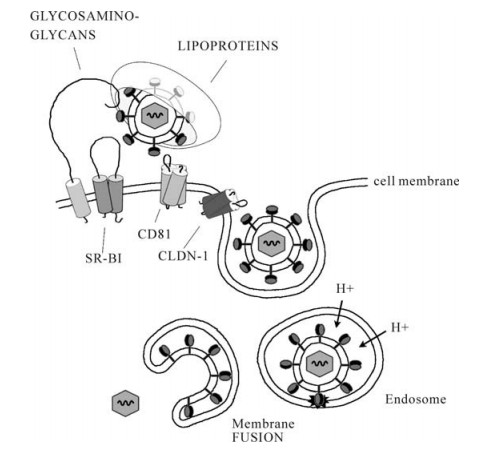
Figure 1. Current model of binding and entry of hepatitis C virus (HCV). HCV is associated with lipoproteins. The lipoprotein/virus particle is captured by cellular binding factors (glycosaminoglycans) and the virus may then be transferred to the entry factors including scavenger receptor B type Ⅰ (SR-BI), tetraspanin CD81 and claudin-1 (CLDN-1). This orchestra of binding events triggers clathrin-mediated endocytosis of the HCV particle. The HCV capsid and genome is released into the cytosol following pH-dependent membrane fusion of the viral surface glycoproteins and the endosomal membrane.
-
The HCV genome encodes a single precursor polyprotein of about 3, 000 amino acids that is cleaved co-and post-translationally into functional structural and non-structural proteins by host and viral proteases including two envelope glycoproteins, E1 and E2. In analogy to other members of the Flaviviridae family, the HCV capsid complexes the viral RNA genome and is thought to adopt a classical icosahedral scaffold in which the two envelope glycoproteins E1 and E2 are anchored to the host cell-derived double-layer lipid envelope (38). E1 and E2 are type Ⅰ transmembrane glycoproteins containing up to 6 and 11 potential glycosylation sites, respectively and forming noncova-lent heterodimers.
Studies of infectious HCV life cycle have been limited by the lack of an efficient cell culture system. Several model systems have thus been developed for the study of defined aspects of the HCV life cycle such as viral entry, replication, assembly and release (for review see (5)). Recombinant HCV envelope glycoproteins (40), HCV-like particles (HCV-LPs) (4, 13, 53) and retroviral HCV pseudotypes (HCVpp) (10, 24) have been successfully used to analyze virus attachment and entry. Most recently, efficient in vitro model systems for the production of infectious recom-binant virions (HCVcc) have been described (32, 60, 52). Using these model systems, it could be demon-strated that envelope glycoproteins E1 and E2 are critical for host cell entry.
-
Using various model systems for HCV-host interaction, several host entry factors have been identified. These include CD81 (40), the low-density lipoprotein (LDL) receptor (1), highly sulfated heparan sulfate (6, 27), scavenger receptor class B type Ⅰ (SR-BI) (42), and claudin-1 (CLDN-1) (19).
Heparan sulfate is an important cellular binding molecule for several viruses and may serve as the initial docking site for HCV attachment. In fact, incubation of HCV-LPs and HCVpp with heparin -a structural homolog of highly sulfated heparan sulfate-reduced HCV-LP and HCVpp binding to human hepatoma cells resulting in a decreased internalization of theses particles (6, 7). Most recently, using the HCVcc system, it could be confirmed that heparan sulfate plays an important role for HCV attachment to its target cell (27).
Tetraspanins are widely expressed proteins that regulate cell morphology, motility, invasion, fusion and signalling (23). Two tetraspanins-CD81 and CLDN-1 have been shown to represent key factors for HCV infection (19, 40). Anti-CD81 antibodies as well as a soluble form of the CD81 extracellular loop have been shown to inhibit HCVpp and HCVcc entry into Huh-7 hepatoma cells and human heptocytes (10, 24, 32, 52, 59, 60). Expression of CD81 in hepatoma cell lines that are resistant to HCVpp and HCVcc infection conferred susceptibility to HCV infection (2, 12, 59). In addition, it has been demonstrated that CD81 expression levels on hepatoma cells correlate with HCV infectivity (2, 26). CLDN-1 is highly expressed in the liver but also in other tissues. Expression of CLDN-1 in non-hepatic 293T cells renders them susceptible to HCVpp entry (19). In addition, overex-pression of this molecule in CD81-deficient HepG2 hepatoma cells did increase their HCV permissivity, suggesting that CLDN-1 is not an alternative entry pathway to CD81 (19). Kinetic studies showed that CLDN-1 acts at a post binding step after HCV interaction with CD81 (19).
SR-BI is a 509 amino acid glycoprotein with a large extracellular loop anchored to the plasma membrane at both the N-and C-termini. SR-BI is highly expressed in liver and steroidogenic tissues (28) as well as human monocyte-derived dendritic cells but not on any other peripheral blood mononuclear cells (55). It has been shown that physiological SR-BI ligands, such as HDL or oxLDL, can modulate HCV infection: HDL and oxLDL have been shown to enhance and inhibit HCVpp entry, respectively (11, 49, 50), whereas both HDL and LDL inhibited HCV replication in human hepatocytes infected with serum-derived HCV (36). The important role of SR-BI in productive HCV infection has been confirmed using the HCVcc system (25, 58).
The apolipoprotein B (apoB)-containing LDL and apolipoprotein E (apoE)-containing very low-density lipoproteins (VLDL) are the major LDLR ligands. As HCV is able to associate with LDL and VLDL in serum (3), the LDLR was suggested to be a putative HCV receptor candidate. The LDLR has been shown to internalize serum-derived HCV by binding virus-LDL particles (1). Anti-LDLR antibodies as well as anti-apoB and apoE antibodies were able to inhibit HCV endocytosis (1, 10, 54). It could also be demon-strated that LDLR plays a role in an early step of serum-derived HCV infection of primary human hepatocytes (36). However, studies using the HCVpp system where HCV is not associated with lipoproteins suggest that LDLR does not appear to play a role for infection of Huh7 cells with HCVpp (10). Further studies using HCVcc and human hepatocytes will allow more insight into the role of LDLR in HCV infection.
Despite the numerous experimental data demon-strating the importance of the above described receptors in HCV infection, none of these molecules has a liver-specific expression profile as it would be expected for receptors of a hepatotrophic virus. Moreover, all HCV permissive cell lines identified so far express CD81, SR-BI and CLDN-1 and are of hepatic origin but various cell lines of non-hepatic origin expressing these receptors are non permissive for HCV (9, 10, 12, 59), suggesting that additional liver specific factor (s) are required for HCV infection.
Viral determinant of viral entry: envelope glycoproteins
Host entry factors for HCV infection
-
The recent development of tissue culture model systems for the study of HCV infection (HCVpp, HCVcc) has finally allowed to functionally charac-terize antibody-mediated neutralization in HCV infected patients (for review see (57)). Using the HCVpp model system, two studies have demonstrated that neutralizing antibodies are induced in the early phase of infection by patients who subsequently clear the virus (39) or control viral infection (30). In a well characterized single-source outbreak of hepatitis C, viral clearance was associated with a rapid induction of neutralizing antibodies in the early phase of infection. In contrast, chronic HCV infection was characterized by absent or low-titer neutralizing an-tibodies in the early phase of infection (39). These results suggest that a strong early broad neutralizing antibody response may contribute to control of HCV in the acute phase of infection and assist cellular immune responses in viral clearance. Furthermore, experimental data obtained in animal models have demonstrated that immune control of poorly cyto-pathic viruses, such as lymphocytic chorio-meningitis virus (LCMV) requires a collaboration of both the cellular and humoral arms of the immune system (20). Applying these findings to HCV infection -another prototype of persistent-prone non-cytopathic viruses -it is conceivable, that both cellular (15, 46, 47) and neutralizing responses (30, 39) may contribute to control of HCV infection during the very early phase of viral infection.
Patients who do not clear the virus develop high-titer and even cross-neutralizing antibodies during the chronic phase of infection (9, 34, 39, 45, 51, 52). Paradoxically, these antibodies are not able to control HCV infection. Viral escape from antibody-mediated neutralization in these patients may occur on several levels: (ⅰ) HCV exists as a quasispecies with distinct viral variants in infected individuals changing constantly over time (9); (ⅱ) the interplay of HCV glycoproteins with high-density lipoprotein and SR-BI has been shown to mediate protection from neutra-lizing antibodies present in sera of acute and chronic HCV-infected patients (11, 18); and (ⅲ) as shown for other viruses such as human immunodeficiency virus (HIV), escape from neutralizing antibodies may occur through a combination of different mechanisms, for instance point mutations, insertions/deletions or chan-ges in glycosylation patterns of the viral envelope (21) or conformational masking of receptor binding sites following envelope-antibody interaction (29) preven-ting neutralizing antibody binding (44).
-
In other viruses, such as HIV, viral entry has been shown to represent an important target for antiviral strategies (16). The lectin cyanovirin-N (CV-N) has been discovered as an active compound against HIV and was then shown to present antiviral activity against other enveloped viruses (16, 37). This antiviral activity results from interactions between CV-N and high-mannose oligosaccharides on viral envelope gly-coproteins (43). HCV envelope glycoproteins are highly glycosylated and contain oligomannose glycans. It has been shown that these oligomannose glycans interact with CV-N resulting in HCV antiviral activity by blocking HCV entry into target cell (22). Another approach to target HCV attachment might be the development of heparan-derived molecules, as heparin has been shown to potently inhibit HCV E2, HCVpp, HCV-LP as well as HCVcc binding to hepatoma cells (6, 8, 27). The systematic generation and screening of heparan sulfate-like molecules and semisynthetic deri-vatives is already explored as an antiviral approach against dengue virus infection (31).
Insights into the molecular mechanisms of HCV fusion are just about to arise and molecules likely to interfere with HCV penetration have not yet been described. As HCV enters the host cell through endocytosis and requires low pH for delivery of HCV genome, agents preventing the acidification of endo-somal compartments, such as chloroquine, are able to prevent infection (14, 48). Peptide-based fusion inhibitors have already been established for the treatment of other viral infections such as HIV infection. Enfuvirtide which blocks HIV fusion to host cells is the first compound of this family approved for clinical use (41). As for other infectious diseases, it might be preferable to target viral proteins rather than host cell components because of potential adverse effects resulting form interference with normal cell functions. The optimal entry inhibitor would block viral binding sites on receptors without affecting functional physiological ligand binding. Drugs tar-geting the viral entry could increase the impact of the current antiviral combination therapy using interferon and nucleoside analogs.
Target of host neutralizing responses
Entry inhibitors as novel antiviral therapeutics
-
Following the development of novel cell culture models for HCV infection our understanding of the HCV entry process and mechanisms of virus neu-tralization has been markedly advanced. Viral entry has important clinical implications since it is a target of host neutralizing antibodies and antiviral strategies. Both viral and host cell components involved in virus entry may serve as targets for the development of novel HCV entry inhibitors potentially overcome the limited efficiency of current antiviral therapies.














 DownLoad:
DownLoad: