-
Hendra virus (HeV) and Nipah virus (NiV) are emerging zoonotic paramyxoviruses responsible for repeated outbreaks in Australia, South Asia, India and Bangladesh. HeV and NiV are closely related and share similar genomic sequence and organization (21) as well as significant antigenic homology (10). The henipaviruses are also distinguished among the paramyxoviruses by their broad species tropism and highly pathogenic nature (reviewed in 4). The genus Henipavirus was created in 2002 to accommodate these 2 novel and closely related specimens among the Paramyxoviridae family of negative sense RNA viruses.
HeV was first identified in 1994 as the etiologic agent of a severe and often fatal acute respiratory disease among horses during two nearly simultaneous yet independent outbreaks in Queensland, Australia (reviewed in 20, 21). During the course of these epidemics, HeV was also transmitted to three human caretakers, two of which died. A few years later, a large multi-state outbreak of severe encephalitis among pig farmers occurred in peninsular Malaysia resulting in the recognition and discovery of NiV in 1998-1999 (reviewed in 14, 19). Here, NiV was primarily transmitted to humans from infected pigs resulting in some 265 cases of human infection with 105 deaths during this initial outbreak. In addition to pigs, several other animals were also infected including dogs, cats and horses. Acute clinical NiV disease in humans typically develops within 2 weeks of infection with the onset of fever, cough, headache, drowsiness, and myalgia (15, 55). Essentially similar findings have been observed in cases of HeV infection, and several animal models have been reported which display varying aspects of pathology that are reflective of human infections (reviewed in 4). Viral infection predominates in the respiratory system and subsequently spreads to multiple organ systems via a hematogenous route (reviewed in 21). Mortality is the result of severe pulmonary pathology and/or viral encephalitis.
Since these initial outbreaks, both viruses have repeatedly re-emerged. HeV outbreaks have occurred in Australia in 1994, 1999, 2004, 2006, 2007 and 2008, always involving horses. There have been three additional confirmed human infections, one in 2004 and two in 2008 with one fatality in the most recent outbreak (reviewed in 19) (1, 2, 40). NiV has caused outbreaks involving hundreds of human cases since its discovery with 9 recognized occurrences in Bangladesh and India since 2001, the most recent in 2008 (reviewed in 19) (3, 26, 30). Several of these recent NiV outbreaks have been associated with higher case fatality rates (~75%), increased incidence of acute respiratory distress syndrome along with neurological disease, and evidence of person-to-person transmission with direct transmission of the virus from natural reservoirs to humans via contaminated food sources (24, 26, 36).
The preponderance of evidence has implicated frugivorous bat species commonly known as flying foxes (order Chiroptera, family Pteropodidae, genus Pteropus) as the principle host reservoir of the henipaviruses (reviewed in 4). These bats are widely distributed throughout South Asia, Oceania, and as far west as Madagascar (22). Serological studies have shown evidence of henipavirus specific antibody or other indications of infection among several bat species from various geographic locations (reviewed in 4). Most recently, serologic evidence of henipaviruses in bats has been demonstrated in West Africa and China, bringing the total number of species to 24 from 10 genera of bats (27, 35). Evidence to date suggests that there are at least three distinct lineages of NiV: Malaysia, Bangladesh (25) and Cambodia (47) (reviewed in 21). It likely that when henipavirus isolates are obtained and characterized from the recently identified reservoirs in Madagascar and China there will be some additions to the NiV or HeV lineages or perhaps even one or more new viral species belonging to the henipavirus genus. These results indicate that the henipaviruses or henipa-like viruses are widespread and present a potentially significant zoonotic disease risk for a large human population. There are currently no approved therapeutics or vaccines available for the prevention or treatment of henipavirus infection and they are classified as biosafety level 4 (BSL-4) pathogens and select agents.
HTML
-
Henipaviruses enter their host cells through a pH-independent fusion mechanism mediated by the cooperative action of their attachment (G) and fusion (F) glycoproteins. These structural glycoproteins are the only viral determinants found in the membrane envelope of the virions and both F and G are required to facilitate membrane fusion (11, 12); unlike some members of the Pneumovirinae and Paramyxovirinae subfamilies which can mediate membrane merger with only their F glycoprotein when it is over expressed (reviewed in 32). Paramyxovirus attachment glycoproteins are designated as either a hemagglutinin–neuraminidase protein (HN), hemagglutinin protein (H), or a glycoprotein (G) when the glycoprotein lacks both hemagglutinating and neuraminidase activities (reviewed in 32). Paramyxovirus attachment glycoproteins are type Ⅱ membrane proteins with the molecule's amino (N)-terminus oriented towards the cytoplasm and the protein's carboxy (C)-terminus facing the extracellular environment (reviewed in 8). The other major envelope glycoprotein is the fusion (F) glycoprotein which is a trimeric class Ⅰ fusogenic protein containing two heptad repeat (HR) regions and a hydrophobic fusion peptide (reviewed in 8).
Paramyxovirus attachment glycoproteins, including the henipavirus G glycoprotein, contain a globular head domain, a stalk region, transmembrane domain, and a short cytoplasmic tail (9, 57) The globular head domain of the attachment glycoprotein contains all of the enzymatic activities of the molecule and mediates viral adsorption with the target cell (reviewed in 39). The stalk domain contains important cysteine residues required for the formation of disulfide linkages creating dimeric oligomers (33, 57). Homodimeric oligomers further associate to form tetrameric complexes of the attachment glycoprotein (9, 33)
The F glycoprotein is directly involved in mediating membrane merger (reviewed in 8). F is synthesized as a precursor molecule (Fo) that is cleaved into an active form by host cell cathepsin proteases prior to incorporation in the virion (42, 43). The cleavage products, F1 and F2, remain associated by a disulfide bridge (reviewed in 32). F1 possesses an HR element located adjacent to the fusion peptide and is referred to as the N-terminal heptad or heptad repeat A (HRA) as well as a second HR region proximal to the transmembrane domain referred to as the C-terminal heptad or heptad repeat B (HRB). Experimental evidence indicates that the trimeric F glycoprotein spike is associated with the tetrameric G glycoprotein spike prior to receptor binding, similar to other well-characterized paramyxoviruses (6).
-
Molecular characterization of paramyxovirus envelope glycoproteins has led to the development of a theoretical model for the membrane fusion process. Henipavirus adsorption to host cells is mediated by the interaction of G with one of the alternate viral receptors, ephrinB2 or ephrinB3 (7, 41). EphrinB2 and B3 are members of a large family of surface expressed glycoprotein ligands that bind Eph receptor tyrosine kinases (18, 44, 46). The Eph receptors and their ephrin ligand partners make up an important group of bi-directional signaling molecules. These molecules are known to participate in a variety of cell-cell interactions including those of vascular endothelial cells and are the modulators of cell remodeling events, especially within the central nervous system (CNS). EphrinB2, in particular, is highly conserved and widely expressed in animal tissues (reviewed in 28, 54, 59). Its identification as a major receptor for the henipaviruses has helped clarify the broad species and tissue tropisms of NiV and HeV as well as their pathogenic processes observed in both humans and animal hosts.
The recent crystal structures of NiV G in complex with receptor indicate the receptor binding domain (RBD) is conformation-dependent and consists predominately of 2 regions: a large docking region of polar residues near the rim of the globular head and a distinct channel which accepts residues of the B class ephrin G-H loop (13, 56). Interestingly, detailed analysis of the channel revealed a small hydrophobic pocket homologous to the 2-deoxy-2, 3-dehydro-N-acetylneuraminic acid (sialic acid) binding site of human parainfluenza virus 3 (hPIV-3) HN (56). In addition, sequence analysis of the HeV G glycoprotein shows the molecule is more similar to hPIV-3 HN than measles virus (MeV) H (57).
Earlier site-directed mutagenesis or examination of the crystal structure revealed residues W504, E505, Q530, T531, A532, E533, N557 of NiV G and residues D257, D260, G439, K443, G449, K465, and D468 of HeV G as important in the interaction of G with the ephrin receptor molecules (6, 23, 56). Since both viruses use the same B class ephrin receptors, and given the significant level of structural conservation and similar susceptibilities to certain monoclonal antibodies (mAb) in virus neutralization, it is likely the majority of particular residues within G essential for the interaction with the receptor will be similar for both viral species. Further, the crystal structure of NiV G in complex with ephrinB3 and B2 confirms the importance of the B class Ephrin G-H loop for binding within the channel of the NiV G globular head (13, 56). In addition, the importance of the G-H loop as the principle site of interaction between G and the ephrins is similar to reports showing the importance of this loop among the B class ephrins in binding to their Eph receptor partners.
Following virus adsorption, the F glycoprotein mediates the merger of the viral and host cell membranes. Conformational changes in the F trimer produce an extended molecule exposing the fusion peptide which inserts into the target cell membrane (reviewed in 31). After a physical link has been established between the membranes, the two HR domains undergo significant conformational rearrangements whereby the HRB domains fold into the grooves of the trimeric HRA domain core forming a hairpin bundle of α-helices known as the 6-helix bundle. The formation of the 6-helix bundle structure is concomitant with membrane merger and appears to drive the fusion process. Once fusion occurs between the virion and host cell membranes, the virion contents are released into the cytoplasm initiating virus replication.
Molecular characterization of the fusion process has resulted in significant refinements of the theoretical model of membrane fusion mediated by the paramyxoviruses. In particular, studies have shown that specific peptides mimicking the HR regions are capable of inhibiting fusion at defined steps (48). These experiments point to specific conformational changes in F that proceed in a defined sequence. The solution structures of both pre-and post-fusion forms of two paramyxovirus F glycoproteins were found to be consistent with HR peptide fusion inhibition studies and have further detailed the conformational changes in F in relation to the model of paramyxovirus membrane fusion (reviewed in 31). However, major gaps in our understanding of the paramyxovirus fusion process remain, particularly in the nature of the interaction between the attachment and fusion glycoproteins and precise details of the receptor-induced processes leading to the activation and conformational changes in the F glycoprotein
-
Many early studies clearly demonstrated the necessity for both the attachment and fusion glycoproteins to promote efficient membrane fusion among the paramyxoviruses. Expression of either glycoprotein without its envelope glycoprotein partner results in the absence of detectable fusion with few exceptions (32, 33). In addition, only envelope glycoproteins from the same or highly related viruses, such as the henipaviruses, mediate fusion when co-expressed in a heterotypic manner (12). We have previously shown the co-precipitation of G and F in the absence of receptor suggesting these glycoproteins interact and remain associated on the surface of the virion prior to adsorption (6). Several reports have implicated regions in the stalk domain and/or the β2 sheet region of various attachment glycoproteins as important elements of the molecule in its interaction with F (17, 37, 49, 50, 52, 53). However, to date, these findings have not definitively shown the precise molecular elements involved in their association and uncertainty remains as to the specific regions in both F and the attachment glycoprotein which are involved.
Certain isoleucine residues in the stalk domain of the attachment glycoprotein are thought to form a HR-like structure important in the interaction between the attachment and fusion glycoproteins (50). Mutations made in this region of the Newcastle disease virus (NDV) HN have been shown to alter both the interaction of HN with F and the capacity of the viral proteins to promote fusion (38, 50). Mutations in the analogous region of MeV H also disrupt fusion; however, do not disrupt the interaction of H with F (16). As a result, it has been proposed that paramyxoviruses modulate F activation differently based on the nature of the target viral receptor (i.e. a sialic acid moiety or protein) (29). Recently, we reported identification of a series of isoleucine residues in the stalk domain of HeV G that appear important in the G glycoprotein's structure and fusionpromotion activity (5) Comparison of the amino acid sequence of NiV G and HeV G indicates these isoleucine residues are identically conserved and form an imperfect HR-like arrangement (5). Individual sitedirected mutagenesis of each isoleucine residue was performed and although 9 of 12 of these HeV G mutants were expressed on the cell surface and retained receptor-binding competence, they were completely defective in their fusion-promotion activity. Additional analysis of these defective cell-surface expressed mutants revealed that they were differentially glycosylated with complex oligosaccharides and migrated as a slightly higher molecular weight species. However, analysis of the HeV G isoleucine mutants produced in the presence of 1-deoxymannojirimycin, HCl indicated that inhibiting the addition of high molecular weight mannose species did not alleviate their fusion-promotion defect (5). Co-precipitation studies of these G mutants with F revealed defects in F association in the absence of receptor, suggesting the isoleucine mutations were altering the conformation of G and preventing its association with F.
-
The favored model of paramyxovirus fusion asserts that specific receptor binding induces changes in the attachment glycoprotein which triggers the activation of F allowing fusion to proceed. In fact, structural studies by Takimoto et al. suggested the conformation of the outer face of the NDV HN globular head varied when bound to sialic acid (51). However, subsequent crystal structures from other paramyxovirus attachment glycoproteins have not demonstrated major conformational variations when comparing receptor bound and unbound structures (13, 34, 56, 58). In particular, the structure of NiV G in complex with ephrinB3 shows remarkably little alteration in the conformation when compared with the structure of unbound NiV G (56). These structures show alterations in the conformation are principally restricted to the binding pocket and interface with ephrinB3 (56). It seems unlikely that these minor conformational differences alone would be sufficient to affect the interaction of F and G and the resultant triggering of the conformational alterations in F leading to membrane fusion.
Our data (5 and Hickey and Broder unpublished) has indicated that the antigenic characteristics of HeV and NiV G are altered following receptor binding. For example, comparing the amount of G precipitated in the presence and absence of ephrinB2 receptor shows G specific mAbs (targeting 5 distinct epitopes; Hickey and Broder unpublished) precipitate more glycoprotein in the presence of receptor (complexes of G-ephrinB2) than in the absence of receptor (Fig. 1A). Interestingly, several mAbs recognize full-length native G only in the presence of bound ephrinB2 indicating G undergoes significant antigenic modification following receptor binding. The inconsistency of these data with observations made from examination of the crystal structures are likely due to the differences in the conformation of the native oligomeric tetramer of G in comparison to the monomeric G-ephrinB3 and B2 structures. Indeed, when comparing the amount of recombinant soluble G (sG) in similar precipitation experiments with these same mAbs, the disparities that are observed with full-length G are significantly reduced or absent (Fig. 1B). In addition, mAbs which recognize full-length G only in the presence of receptor were able to bind both the receptor-bound and receptor-unbound forms of sG at near equivalent levels. While sG has been thoroughly characterized and shown to closely mimic the full-length protein it is principally dimeric and does not form tetrameric oligomers similar to the full-length protein (9). These data strongly suggest that receptor binding induces alterations in the oligomeric complex of the G tetramer rather than major conformational changes in a G subunit monomer. Indeed, some mAbs which bind G better in the presence of receptor also bound the fusion-defective isoleucine residue stalk mutants better but in the absence of any receptor (5). Thus, these data indicate that the stalk mutants appear to adopt a postreceptor bound conformation; that is a pre-triggered form of G which can no longer promote F-mediated fusion even after receptor binding (5). Together, these data support a model of paramyxovirus fusion whereby F activation and subsequent fusion follows a proteinreceptor induced change in G, perhaps a result of modifications of how the dimers associate. A cartoon demonstrating this model is show in Fig. 2.
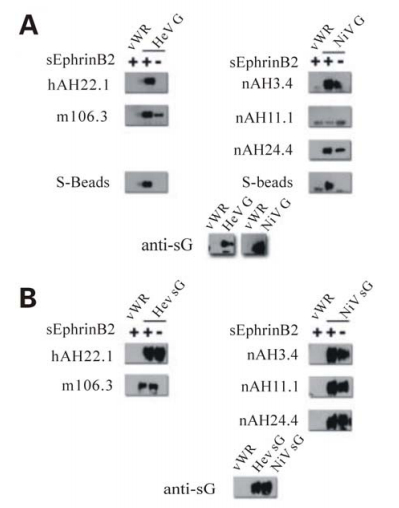
Figure 1. Differences in the pattern of MAb recognition of G in the presence or absence of soluble receptor suggests G undergoes changes in conformation or oligomeric form following adsorption. Supernatant and cell lysates of HeLa-USU cells infected with either wild type (vWR) or recombinant vaccinia virus expressing full-length (A) or soluble (B) Henipavirus G were prepared as previously described (5). 100 μL of cell lysate (panel A) or clarified supernatant (panel B) were incubated with 1 μg of purified soluble s-tagged EphrinB2, overnight at 4 ℃ followed by mAb for 1 h at room temperature. G bound to mAb was precipitated using Protein G sepharose beads washed separated by electrophoresis, and blotted as described previously (5). As described, blots were probed with polyclonal rabbit anti-sG (1:20 000) and developed using horseradish peroxidase conjugated goat anti-rabbit (1:20 000) with the WestPico Chemiluminescent substrate (Thermo Scientific; Rockford, IL).

Figure 2. The model of F glycoprotein activation following adsorption to the host cell. A: Extracellular free virus exhibiting multiple complexes of homotrimeric F associated with homotetrameric G targets the host cell displaying EphrinB2 or EphrinB3 on the surface. B: The globular head of G binds the extended G-H loop region of the B class Ephrin molecule. C: Adsorption induces changes in G, represented here by the dissociation of the G oligomer into dimers linked by a disulfide bridge (solid black line). D: G oligomer dissociation frees F from the complex allowing the conformation of F to assume an activated state. E: Activation of F reveals the hydrophobic fusion peptide which is inserted in the host cell membrane initiating the events leading to membrane merger.
Iorio et al proposed that paramyxoviruses may mediate the activation of F by distinct mechanisms based on the nature of the receptor, carbohydrate or protein (29). Our data indicate the henipaviruses facilitate fusion activity via a mechanism consistent with NDV, which binds sialic acid, and is proportional to the extent of G and F association (5, 29, 38). However, the fusion activity of MeV, which also utilizes a protein receptor, is inversely related to the extent of the association of the envelope glycoproteins (16, 45). The incongruence of our data with the model proposed by Iorio et al. can perhaps be somewhat clarified by examination of the NiV G crystal structures recently published. These structures show the site of interaction with the B class ephrins is located on G in an analogous position to the sialic acid binding site of NDV (5, 56). Whereas, the RBD described for MeV H is located in a position on the globular head further from the dimer interface than found with NDV HN (29). It is therefore plausible that the divergent mechanisms described by Iorio et al. are likely not based on the nature of the receptor recognized by an attachment glycoprotein, but rather the nature and location of the binding site on the molecule itself.
-
Recent findings from studies in several paramyxovirus systems have afforded a better understanding of the binding and infection process and the refinement of the model of henipavirus and paramyxovirus mediated membrane fusion. These data support a multi-step process of attachment glycoprotein receptor binding and conformational changes in its oligomeric structure that trigger F activation leading to membrane fusion. Activation of F results in a sequential series of conformational changes including the insertion of the F fusion peptide into the target cell membrane and subsequent refolding of F from a prefusion form to the post-fusion 6-helix bundle driving membrane merger. Additional research will be required to delineate the epitopes targeted by some of the mAbs discussed here, which will aid in identifying regions of the G glycoprotein that are altered following receptor binding. Further, classification of additional functional roles of G should help define the mechanisms of neutralization for different classes of antibodies, such as those mAbs which do not compete with receptor for binding G, as well as aid in defining the precise interactions between F and G. Understanding these functional aspects and details could provide insight in the design of novel therapeutic agents.














 DownLoad:
DownLoad: