-
A DNA fragment of Kaposi's sarcoma-associated herpesvirus (KSHV), also termed human herpesvirus 8 (HHV8), was found by representational difference analysis (RDA) in DNA preparation of Kaposi's sarcoma (KS) tissue from an HIV-infected individual (5). Since then, the causal link between KSHV and KS has been well established. KSHV is consistently found in all forms of KS: (classic, endemic, AIDS associated and transplantation associated). KSHV has also been observed to be associated with primary effusion B cell lymphoma and multicentric Castleman's disease(4, 17). The nucleotide sequence of the virus genome comprises~210 kb and consists of≥90 open reading frames (ORFs) (14). By now, there have been many studies around the world investigating the distribution and infection of KSHV in different areas. KSHV is found to have a worldwide occurrence but infection rates vary according to a combination of geographic and behavioral risk factors (15). Seroprevalence of KSHV is shown to be 25% higher in African countries, whereas in the United States, Asia, and Western Europe it is lower than 10% (3, 9, 19). Several methods have been implemented for detecting KSHV, mainly based on serological and molecular diagnosis (6, 7, 12, 16). At least 4 different serologic assays have been utilized to detect the HHV8 antibodies; immunofluorescent assays (IFA), enzyme-linked immunosorbent assays (ELISA), Western blot and immunohistochemistry (IHC). For screening a large amount of sera, the ELISA method is always used in the epidemiologic research. The quality of antigen is essential in ELISA assays; therefore, purified antigen with high quality should be used.
orfK8.1 was identified to be located at nucleotide positions 76 214-76 994 within the HHV8 genome, orfK8.1 codes for a 35 to 37 kDa protein that is most likely located within the envelope of the virus and appears to be a major target for human antibodies (13). In this study, the orfK8.1 was cloned and expressed as a recombinant protein in E. coli., and our data indicates that it possesses good antigenicity and specificity in ELISA detecting KSHV using sera.
HTML
-
TA cloning vector pGM-T was purchased from Qiagen Co, prokaryotic expression vector pQE-80L was purchased from QIAGEN Co, competent cells BL21 (DE3) were purchased from Invitrogen Co.
-
Primer pair (P1: 5'-GTGCGGATCCAATTGTCC CACGTATCGTTC-3', P2: 5'-GGCAGTCGACTTA TGGCACACGGTTACTAGCACC-3') was used for amplifying the N-terminal part of orfK8.1. The genomic DNA was extracted from the KSHV positive cell line BCBL-1. The truncated orfK8.1 was amplified with Primers P1 and P2. PCR was performed over 30 cycles of 94℃ 1 min, 63℃ 1 min and 72℃ 1 min, followed by an extension at 72℃ for 10 min. PCR products were separated by agarose gel electro-phoresis and visualized by ethidium bromide staining.
-
PCR product was inserted into pGM-T vector and then transformed into Top10 competent cells. The recombinant plasmid containing the correct length and the direction of the truncated orfK8.1 gene was identified by Sal Ⅰ and BamH Ⅰ digestion and con-firmed by sequencing. The truncated orfK8.1 gene in the pGMT-orfK8.1 plasmid was removed by Sal Ⅰ and BamH Ⅰ digestion and subcloned into pQE-80L plasmid; the recombinant plasmid was named as pQE80L-orfK8.1
-
The pQE80L-orfK8.1 was inoculated into 5 mL LB medium containing 50 μg/mL ampicillin, incubated at 37℃ until the OD600 was about 0.6, and then IPTG was added to the medium with to a final concentration of 1mmol/L. The culture was further incubated at 30℃ for another 6 h at which point the culture was harvested by 5 000 r/min centrifugation. The harvested culture was resuspended by 10×volumes PBS and ultrasonicated (250w, 3×5 second burst) for 5 minutes. The culture was then added with 20% Triton X-100 to a final concentration of 1%, stirred for 30 minutes at room temperature, 10 000 r/min centrifuged for 10 min. The supernatant and the precipitate was collected separately, mixed with SDS-PAGE Gel loading buffer, boiled for 10 min, then loaded onto 10% SDS-PAGE and electrophoresed at 200 V. To purify the recombinant ORFK8.1, Ni-NTA Agarose was used according to the protocol.
-
Proteins of the whole cell lysate and the purified recombinant ORFK8.1 were separated by SDS-PAGE and transferred by electroblotting onto a polyvinyllidene difluoride membrane. The membrane was washed and blocked with the blocking solution (PBS-T) containing 0.2% Tween-20 and incubated with serum called S558 from a AIDS-KS patient or serum called H14 from a blood donor as the primary antibody. S558 and H14 was characterized as KSHV positive and negative serum respectively (2). The membrane was washed in PBS-T (containing 0.2% Tween-20) and incubated at room temperature for 1 h with anti-human IgG-HRP antibody (1: 10 000 dilution). The bands were detectedusing an Alpha Innotech MultiImageTM system after incubationin Chemiluminescent Substrate (Pierce).
-
ELISA plates were coated with 50 μL purified ORFK8.1 (from 1 μg/mL to 10 μg/mL) in 0.1 mol/L NaHCO3 at pH 9.6 and left overnight at 4℃. A conventional ELISA protocol was used with phosphate-buffered saline containing 0.05% Tween 20 (PBS-T, pH 7.4) for washes, 5% dried skimmed milk in PBS-T (blocking buffer) to saturate plates and to dilute S558 or H14 (from 1: 100 to 1: 1 000), and an alkaline phosphatase conjugated affinity purified goat anti-human IgG, diluted 1 in 1 000 in blocking buffer, followed by 1 mg/mL of nitrophenyl phosp-hate in glycine buffer (0.1 mol/L glycine, 1 mol/L MgCl2, 1 mmol/L ZnCl2, pH 10.4) as substrate. The colorimetric reaction was stopped after 25 min at 37℃ with 100μL 3 mol/L NaOH and read spectrophoto-metrically at 405nm.
-
In order to confirm the sensitivity and specificity of ORFK8.1, sera from 20 KS patients and 50 blood donors that were determined negative for antibodies to ORF65 were used in ELISA assay. 560 sera from blood donors in Hubei province were collected from April of 2004 to April of 2005 (8) and used in the investigation of KSHV seroprevalence. A serum sample with an OD above the averages plus five standard deviations of the negative controls wells was considered as positive for the assay.
Plasmids and bacteria
orfK8.1 gene amplification by PCR
Construction of prokaryotic expression plasmid pQE80L-orfK8.1
Expression and purification of ORFK8.1
Western blot analysis
Optimization of the condition of ELISA
Investigation of KSHV seroprevalence using recombinant ORFK8.1
-
A 538 bp fragment was obtained by PCR and cloned into pGMT-easy vector and sequenced. The orfK8.1 gene fragment was then subcloned into the pQE-80L vector; the recombinant plasmid was named as pQE80L-orfK8.1. After being induced by IPTG for 6 h and identified by SDS-PAGE electrophoresis, proteins at about 26 kDa were expressed in BL21 (DE3) strain transformed by pQE80L-orfK8.1 (Fig. 1). Further separation of supernatant and precipitation indicated that the concentration was very high in the precipitation of the lysed BL21 (DE3) transformed by pQE80L-orfK8.1. High purity recombinant precipi-tation protein was attained after Ni2+-nitrolotriacetic acid resin affinity chromatography purification; analysis using the bandscan software indicated that the purity of the recombinant ORFK8.1 protein was about 95% (Fig. 1).

Figure 1. Expression and purification of KSHV ORFK8.1 protein. 1, The cell lysate of pQE80L-orfK8.1/BL21(DE3) before IPTG induction; 2, The cell lysate of pQE80L-orfK8.1/BL21(DE3) after IPTG induction; 3, The supernatant of the lysed pQE80L-orfK8.1/BL21(DE3) after IPTG induction; 4, The precipitate of the lysed pQE80L-orfK8.1 /BL21 (DE3) after IPTG induction; 5 and 6, Purified ORFK8.1; M, Protein marker.
-
The total protein of lysed BL21 (DE3) transformed by pQE80L-orfK8.1 and the purified recombinant ORFK8.1 were electrophoresed by SDS-PAGE and transferred to a PVDF membrane. The membrane was coated with the KSHV positive serum or negative serum. A specific 26 kDa band occurred in the lanes of the total and purified protein of lysed BL21 (DE3) transformed by pQE80L-orfK8.1 when proteins were incubated with serum from KS patient; no band was seen when proteins were incubated with serum from healthy blood donors (Fig. 2). The result indicates that the recombinant ORFK8.1 has very strong specificity and antigenicity.
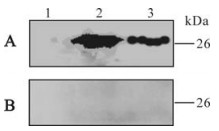
Figure 2. Western blot of recombinant ORFK8.1 protein. A: Reacted with serum from KS patient. B: Reacted with serum from blood donor. 1, The cell lysate of pQE80L-orfK8.1/BL21 (DE3) before IPTG induction; 2, The cell lysate of pQE80L-orfK8.1/ BL21(DE3) after IPTG induction; 3, Purified ORFK8.1.
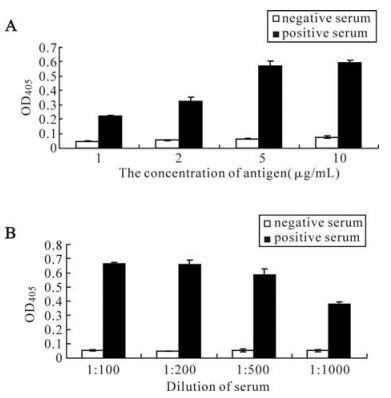
The antigenicity of the recombinant ORFK8.1 was also assessed by ELISA. The recombinant ORFK8.1 was used as antigen to coat the ELISA plates; KSHV positive and negative serum were used as the primary antibody. The ELISA result showed that the optimal antigen concentration was 5 μg/mL (Fig. 3A) and the optimal dilution of the serum was 1: 200 (Fig. 3B). The result confirmed the antigenicity and specificity of the recombinant ORFK8.1 as a diagnostic antigen for KSHV.
-
To examine the KSHV serostatus in the studied subjects, KSHV lytic antigen ORFK8.1 was used to detect specific antibodies. To evaluate the overall specificity and the detection ratio of the assays, 20 classical KS patients provided and confirmed by the Laboratory of Xinjiang Endemic and Ethnic Diseases (Shihezi University) were examined who would usually be 100% positive for ORFK8.1 antibody (10) and 50 healthy subjects which were characterized previously (8) were also included as control. Overall, the KSHV serological assay using recombinant ORFK8.1 could determine all the 20 KS sera as KSHV positive and thus had a combined specificity of 100%.
Using the ELISA assay, KSHV seroprevalence in the general population in Hubei Province was examined. Of 560 subjects, 38 (6.80%) were KSHV seropositive. Among them, 17(5.36%) were male, 21(8.64%) were female. Analysis of the subjects by age showed that KSHV seroprevalence changed with subject age. Under 20, 20-50, and over 50 have 6.06%, 7.59% and 5.80% KSHV seroprevalence respectively (Table 1).

Table 1. KSHV seroprevalence in Hubei general population
Expression and purification of recombinant OR-FK8.1 protein
Identification of the antigenicity of the recom-binant ORFK8.1
KSHV seroprevalence in Hubei province
-
KSHV/HHV-8 is the only human γ2-herpesvirus identified to date and is related to the Epstein–Barr virus (EBV), the only known human γ1-herpesvirus. Approximately 90 open reading frames (ORF) of KSHV have been identified, and over 60 show homology with other rhadinoviruses.
KSHV exhibits a certain infectivity ratio among normal healthy people who are mainly latently infected. Recently, the number of HIV-1 infection in China is rapidly increasing, and infection of HIV-1 promotes the progression of AIDS-associated Kaposi's Sarcoma. Thus, it is necessary to gain greater understanding of the epidemiology of KSHV in China.
Open reading frame K8.1 is located between open reading frame K8 and orf52 of HHV-8. With respect to its relative position in the genome, orfK8.1 is therefore similar to orf51 of herpesvirus saimiri (1), M7 of murine herpesvirus 68 (18), BORFD1 of bovine herpesvirus 4 (BHV-4) (11), and gp220/350 of Epstein-Barr virus (EBV). These genes invariably encode transmembrane glycoproteins that have been shown to be translated from a spliced message whenever examined. Cross-reactivity of ORFK8.1 with other herpesvirus proteins is unlikely, as orfK8.1 is not conserved among known herpesviruses. Accordingly, when ORFK8.1 were used to detect KSHV, no reactivity was observed in sera from patients with EBV primary infection or nasopharyngealcarcinoma (13). The immunogenic protein encoded by reading frame K8.1 will thus be useful for epidemiological studies.
We have cloned a fragment encoding 171 amino acids from KSHV orfK8.1 into the prokaryotic expression vector pQE80L to obtain the expression plasmid pQE80L-orfK8.1. The recombinant ORFK8.1 could be highly expressed in E.coli strain BL21 (DE3), After Ni2+-nitrolotriacetic acid resin affinity chromatography purification, the purity of the recombinant ORFK8.1 could reach more than 95% of the total protein.
We then used Western blot to check the antigenicity and specificity of the expressed recombinant ORFK8.1. As shown in Fig. 2, the 26 kDa bands only appeared when the ORFK8.1 proteins reacted with KSHV positive serum but not with KSHV negative serum. Also, no 26 kDa bands appeared when the total lysate from BL21 (DE3) transformed with control vector was reacted with either KSHV positive or negative serum. ELISA was also conducted to check the antigenicity and specificity of the expressed recombinant ORFK8.1. When different concentrations of the ORFK8.1 were used to coat the ELISA plates, the OD405 values had no significant differences. Thus, 5 μg/mL was chosen as the optimal concentration to be used in the ORFK8.1 ELISA. Different dilutions of serum were also tried, but there was no significant difference between dilutions of 1: 100 and 1: 200; the serum dilution of 1: 200 was therefore used in the ORFK8.1 ELISA.
We used the expressed recombinant ORFK8.1 as antigen to react with 50 healthy sera and 20 classic KS patient sera. The data suggests that the expressed recom-binant ORFK8.1 is a highly sensitive and specific immunoreactive antigen. The fact that the KS sera tested in ELISA exclusively reacted with the recom-binant ORFK8.1 demonstrates that this antigen is important for serological diagnosis of KSHV infection. We then examined the KSHV serostatus in Hubei general population using the recombinant ORFK8.1 as antigen. The overall KSHV seroprevalence is 6.80%, which is consistent with the fact that KSHV sero-prevalence is low in mainland China (8).














 DownLoad:
DownLoad: