-
The invertebrate virus family Dicistroviridae (18, 31), formerly known as the "Cricket paralysis-like viruses" was recognized in 2002 (64). The name "dicistrovirus" refers to the unique dicistronic arrangement of the genome, which has a positive sense genomic RNA (Fig. 1a). The dicistroviruses are similar to other viruses within the "picornavirus-like super-family" which includes Iflavirus and Picornaviridae (18). Dicistroviruses can be distinguished from members of the taxa Iflavirus, Picornaviridae and Sequiviridae in having the structural proteins at the 3'-end of the genome rather than at the 5' end, and by the presence of the intergenic region (IGR). Dicis-troviruses are distinct from members of the family Comoviridae in having one rather than two genomic segments.
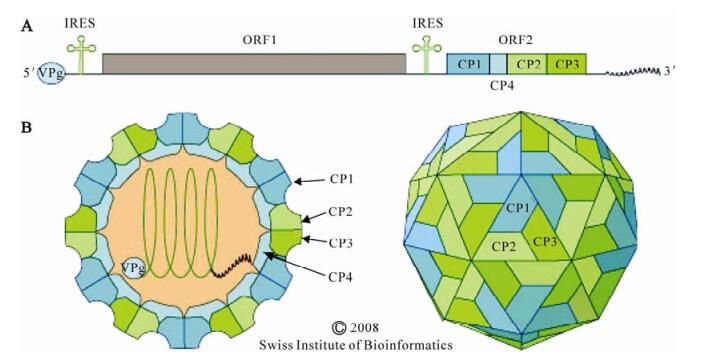
Figure 1. A. Dicistrovirus genome organization. The approximately 8–10 kb RNA genome encodes two polyproteins. ORF 1 encodes the nonstructural proteins: RNA helicase, cysteine protease, and RNA-dependent RNA polymerase. Suppressors of RNA inhibition have also been identified at the N terminus of ORF 1 in some dicistroviruses (see text). ORF 2 encodes the four capsid proteins, CP1 through CP4. The distinct IRESes located in the 5' UTR and intergenic region (IGR), are indicated. The genome has a viral VPg covalently linked at the 5' end and a 3' polyA tract. B. The dicistroivirus virion. Schematic diagrams of a transverse section through the virus (left), and a surface view of the virion (right) are shown. The non enveloped virion of 25 to 30 nm in diameter is icosahedral with T=3 symmetry. The RNA genome attaches to CP4 which lies beneath CP1. Figure reproduced with permission from the Swiss Institute of Bioinformatics.
Dicistroviruses have been isolated from six invertebrate orders from the Insecta and from the decapod crustaceans: The Taura syndrome virus negatively impacts the shrimp farming industry (55, 56). The type species Cricket paralysis virus (CrPV), which is widely distributed in nature (78), is unusual in the breadth of its host range (Table 1). Indeed CrPV has the broadest host range of any invertebrate small RNA virus and also infects a diverse range of cell lines (22).

Table 1. The dicistroviruses
HTML
-
There are currently 12 viruses within the Dicistroviridae with two more (Homalodisca coagulata virus-1, HoCV-1 and Israeli acute paralysis virus of bees, IAPV) pending approval by the International Committee on the Taxonomy of Viruses (ICTV) (Table 1). The Dicistroviridae contains a single genus Cripavirus, named after the type species, Cricket paralysis virus. A proposal to create a second genus "Aparavirus", named after Acute bee paralysis virus (ABPV) is currently pending approval by ICTV. The proposal to divide dicistroviruses into two genera is based on phylogenetic distance (Fig. 2), and on the type of internal ribosome entry site (IRES) present in the IGR. While the IGR IRES of members of the Cripavirus genus has a conserved bulge sequence (UGAUCU and UGC), members of the proposed Aparavirus genus have different bulge sequences (UGGUUACCCAU and UAAGGCUU) and an additional stem loop in the 3' region of the IGR IRES.
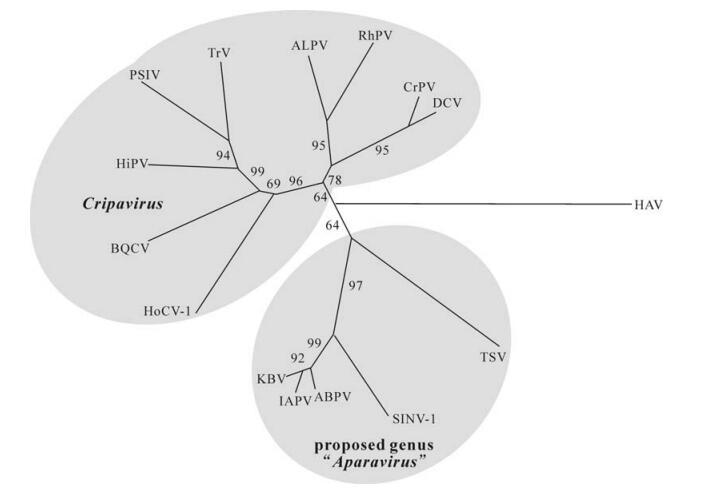
Figure 2. Neighbor joining tree constructed from an alignment of the deduced amino acid sequence of structural proteins encoded by ORF 2 of dicitroviruses. The deduced amino acid sequence for the capsid protein precursor of hepatitis A virus was used for an outgroup. Cripaviruses: ALPV, Aphid lethal paralysis virus, AF536531; BQCV, Black queen cell virus, AF183905; CrPV, Cricket paralysis virus, AF218039; DCV, Drosophila C virus, AF014388; HiPV, Himetobi P virus, AB017037; HoCV-1, Homalodisca coagulata virus-1, DQ288865; PSIV, Plautia stali intestine virus, AB006531; RhPV, Rhopalosiphum padi virus, AF022937; TrV, Triatoma virus, AF178440. Members of the proposed genus "Aparavirus"; ABPV, Acute bee paralysis virus, AF150629; IAPV, Israeli acute paralysis virus, EF219380; KBV, Kashmir bee virus, AY275710; SiNV-1, Solenopsis invicta virus-1, AY634314; TSV, Taura syndrome virus, AF277675. Figure kindly provided by Nobuhiko Nakashima, Chair of the ICTV Dicistroviridae Study Group.
-
The three dimensional structure of CrPV has some similarities to that of vertebrate picornaviruses (92). Dicistrovirus virions appear to be stable both under the highly alkaline gut conditions of the Lepidoptera and at pH 3 (92). The non-enveloped virion of dicistroviruses is approximately 25-30 nm in diameter with an icosahedral, pseudo T=3 symmetry (92). The virions are composed of 60 protomers, each comprised of a single molecule of each of CP2, CP3 and CP1 (Fig. 1b). These three major capsid proteins are generally between 28 and 37 kD. A smaller protein of 4.5 to 9 kD, CP4 is present in some dicistroviruses, and is located on the internal surface below CP1. CP4 provides the interface between the viral capsid and the RNA genome. The lack of the canyon present on the surface-and the pocket in CP1-of picornaviruses, suggests that members of the Dicistroviridae and Picornaviridae use different mechanisms for receptor attachment.
-
The linear, positive sense, ssRNA genome is 8.5 to 10.2 kb with a viral genome-linked protein (VPg) covalently linked at the 5' end and a 3' polyA tract. The 5' UTR is 500 to 800 nt, followed by two open reading frames (ORF 1 and ORF 2) of approximately 5, 500 and 2, 600 nt respectively, separated by about 190 nt in the IGR. ORF 1 encodes the nonstructural proteins (helicase, protease and RNA-dependent RNA polymerase, RdRp) and ORF 2 encodes the structural proteins. The RNA is infectious and serves both as a genome and as mRNA.
Translation of the bicistronic RNA proceeds from the 5' and IGR IRES elements. The IGR IRES, which has a highly conserved secondary structure among dicistroviruses, was rapidly characterized as it provides a remarkable new way for ribosomes to enter an mRNA (28, 29, 103). The IGR IRES can assemble 80S ribosomes without canonical translation initiation factors and initiator tRNA such that translation of ORF 2 begins without the highly regulated, complex process of initiation of translation (84, 102). The absence of an AUG-or other start codon allows the virus to avoid host antiviral translation regulatory mechanisms. The IGR IRES is more active than the 5' IRES resulting in greater accumulation of structural relative to nonstructural proteins (103). The 5' IRES is much less conserved than the IGR IRES and there are no clear structural similarities between the two IRES elements. The 5' IRES is at most 200 nt long, functions in cells from all kingdoms, but until recently has been structurally ill-defined (80, 82). Features of the two IRES elements, which have been reviewed recently (77, 80), are unrelated to any other known IRESes.
The approximately 200 kD and 100 kD poly-proteins produced by ORF 1 and ORF 2 respectively, are cleaved into functional proteins at conserved proteolytic cleavage sites by the ORF 1-encoded protease and by cellular proteases (53, 65, 83).
-
Given the tremendous genetic resources associated with the vinegar fly, Drosophila melanogaster, the Drosophila C virus (DCV), has been used extensively to investigate virus replication. This virus-host combination was used to demonstrate that dicistrovirus entry occurs via clathrin-mediated endocytosis (16). Following entry of the virus into the cell the virus uncoats and releases the genomic RNA into the cytoplasm. Infection results in remodeling of the Golgi apparatus and production of 115 nm diameter cytosolic vesicles mediated by the coat protein complex I (COP I) and fatty acid biosynthesis (17). The viral RNA replication complex associates with the virus-induced vesicles for RNA replication. As for picornaviruses, the 5' VPg protein is thought to prime RNA synthesis, and CAP-dependent translation of cellular mRNAs is inhibited, favoring translation of viral mRNAs (15). Expression of the ORF 1 polyprotein products is required for replication of genomic RNAs in the cytoplasm because ORF 1 encodes the replication enzymes including RdRp. Negative-sense complementary ssRNA are synthesized using the genomic RNA as template and new genomic RNAs are synthesized using the negative-sense RNA as template. The IGR IRES is activated as a result of increased availability of 40S ribosomal subunits following the decrease in cap-dependent translation (32). The ORF 2 polyprotein is then produced and proteolytically cleaved. Assembly of dicistrovirus particles is poorly characterized although the crystal structure of the CrPV virion has been determined (92). When in abundance, virions form large paracrystalline arrays in the cytoplasm of infected cells.
Through a genome-wide RNAi screen using 21, 000 dsRNAs (representing 91% of the predicted genes in Drosophila) the ribosome was identified as limiting for viruses such as DCV with internal ribosome entry sites (15). High levels of ribosomes appear to be required for efficient translation from an IRES, and inhibition of ribosomal function resulted in refractoriness to DCV infection. Modulation of host ribosome activity represents a novel approach for antiviral therapeutics for IRES-dependent viruses of medical importance, such as polio, Hepatitis C and rhinovirus.
Some dicistroviruses, such as CrPV are lytic, while others, such as DCV, are not. The non-lytic viruses can persistently infect host cells without causing obvious cytopathology. Cell lysis results in virus release. In the absence of cell lysis, viral RNAs may spread from cell to cell.
Dicistroviruses may be transmitted horizontally per os, and from females to males (35) and vertically by transovum (79) or transovarial transmission (25, 40). Some viruses such as CrPV and DCV are only transmitted horizontally. Virus particles are shed in the feces of infected insects, providing a source for infection of other insects. Rhopalosiphum padi virus, (RhPV) is somewhat unique in being transmitted horizontally via the plant: RhPV circulates within the phloem of the host plant, thereby using plants as passive reservoirs (34). RhPV is one of few insect viruses known to be plant transmitted (72, 98) but this method of transmission is likely to apply to other dicistroviruses with hemipteran hosts.
-
Several dicistroviruses including CrPV, and DCV were characterized following major crashes of host insect laboratory colonies. Most dicistroviruses result in subtle disease such as reduced longevity and fecundity of infected individuals, while others result in relatively rapid paralysis. Many dicistroviruses primarily infect the gut tissues (RhPV; Aphid lethal paralysis virus, ALPV; Solenopsis invicta virus-1, SINV-1; Himetobi P virus, HiPV). CrPV also infects fat body and tracheae and virus particles were also detected in muscle tissue of the olive fruit fly, Dacus oleae (57). Under certain conditions, CrPV shows increased neurotropism, which results in obvious paralysis. ALPV has also been reported to occur in neural tissue during late stages of infection in the aphid R. padi (40), and Triatoma virus (TrV) can cause paralysis of the host reduviid bug (68).
Relatively little research has been done on the impact of dicistrovirus infection on the ecology and population dynamics of the invertebrate host, beyond the obvious impact of dicistrovirus-induced epizootics (52). One exception to this was investigation of the impact of infection with RhPV on the aphid host (8). RhPV decreases longevity and fecundity of the aphid host (25). Aphids are normally attracted to the odour of healthy aphids but aphids infected with RhPV were not attracted by the presence of healthy aphids, and did not respond to methyl salicylate, which denotes host plant suitability (8). In addition, RhPV -infected aphids were more sensitive to alarm pheromone than uninfected aphids, and infected aphids were more susceptible to attack by the predatory ladybird Coc-cinella septempunctata, and the parasitoid Aphidius ervi (8). Clearly dicistrovirus infection has far-reaching impacts on host physiology with associated alteration of the ecology of the host.
-
To investigate RNA viral gene expression, gene function, and genome replication a reverse genetic system is necessary. This requires a cDNA clone from which infectious RNA can be transcribed. While production of infectious transcripts of Black queen cell virus (BQCV) had been reported (9), an infectious clone of a dicistrovirus was not available until recently (11). While the production of infectious clones of RNA viruses can be notoriously difficult, they provide invaluable tools for both fundamental research and practical applications (69, 75, 93). The dicistrovirus infectious clone was constructed for RhPV, which was first isolated from the bird cherry-oat aphid and was the first pathogenic virus from a hemipteran host to be characterized (25).
To test an infectious clone, a cell line capable of being infected by viral nucleic acid was necessary. In the absence of aphid cell lines (43, 76), two hemipteran cell lines that support RhPV replication were identified (12). Replication was most efficient in the glassy-winged sharpshooter cell line, GWSS-Z10 (12, 50). Transfection of GWSS-Z10 cells with the RhPV transcript of the full length cDNA clone elicited cytopathic effects, ultrastructural changes, and accumulation of progeny virions, consistent with virus infection. Virions from transcript-infected cells were infectious in aphids. The availability of an infectious clone of RhPV will facilitate study of fundamental dicistrovirus biology through the ability to engineer specific mutations into the viral genome (75).
-
Research on dicistroviruses that infect hemipteran (sap sucking) or hymenopteran (e.g. bee) hosts has been challenged by the absence of cell lines capable of supporting virus replication. For reasons that are not well understood, it has not been possible to culture cell lines derived from bees or from aphids (43, 76). In contrast CrPV infects a wide variety of insect cell lines, DCV infects D. melanogaster cell lines (20, 88), and RhPV infects two hemipteran cell lines (12). Indeed one of the major reasons that CrPV and DCV have been so well studied, is that they can both be readily titered and plaque purified (66, 85-87).
To bypass the absence of an aphid cell line, a baculovirus expression vector system has been used for production of a full length clone of the RNA genome of RhPV. The resulting virions were infectious in aphids (73). This is one of few examples of baculovirus expression of a heterologous infectious virus (36, 51), and the first example of baculovirus-expressed virus being infectious in its natural host. Interestingly, more than 100 nt of nonviral sequence at each end of the RhPV clone were maintained during passaging from aphid to aphid for 32 days. Similar toleration of nonviral bases has been reported for other RNA viruses of invertebrates (3, 13, 26, 51). The ability to produce infectious dicistroviruses using the baculovirus expression system also allows for large-scale in vitro virus production, which would be an asset for the use of dicistroviruses for insect pest management (2, 47).
-
D. melanogaster has been studied extensively for delineation of various physiological pathways including host-pathogen interaction (54). The dicistroviruses DCV (46), and CrPV have been used for analysis of virus uptake into cells, and the host immune response to virus infection. DCV is commonly associated with laboratory cultures and wild populations of Drosophila. Affymetrix gene chips were used to examine Droso-phila gene expression 24 hours after per os infection with DCV (81). Eleven genes were induced in response to infection by DCV, including several anti-microbial peptides (attacin A, cecropins A1 and A2, and Drosomycin).
In addition to an inducible response, these studies have demonstrated a cell defense mechanism based on RNA interference (RNAi) in protection of D. melano-gaster against RNA virus infection (33, 99, 100). Suppressors of RNAi have been detected at the N terminus of ORF 1 of CrPV and DCV (99, 100). Although CrPV and DCV share 58% amino acid identity in ORF 1, the sequences of these two suppressors of RNAi are completely different. The nuclease Argonaute 2 (Ago-2), which is the central catalytic component of the RNA-induced silencing complex (RISC), was shown to be essential for antiviral defense against both DCV and CrPV. Flies defective in Ago-2 expression, showed a significant increase in viral RNA accumulation, a 1000-fold increase in virus titer, and increased mortality rate (99).
Three different cell signaling pathways are implicated in humoral immune defense against viruses, the Toll, Imd and Jak-STAT pathways (91). Infection with DCV induced a set of genes distinct from the Toll and Imd pathways that are involved in the insect host response to other RNA viruses (91), implicating the third, evolutionarily conserved innate immunity pathway Jak-STAT in defense against viruses (30). DCV injection induces transcription of some 150 genes. A subset of these genes is regulated by the kinase Jak and transcription factor STAT. Flies with mutations in the gene hopscotch, which encodes the Jak kinase of Drosophila (1), show increased susceptibility to infection by DCV (30, 42, 100). However, the most abundant gene product in the Jak-STAT pathway, Vir-1, had no effect on DCV infection (30). Furthermore, many of the Jak-STAT responsive genes were expressed in non-immune, non-infected tissues. These results provide evidence for the presence of a novel antiviral mechanism in insects (17, 30).
Wolbachia pipientis is a gram-negative, obligate intracellular bacterium that is maternally transmitted in more than 20% of insect species including D. melanogaster. The presence of W. pipientis was found to delay DCV and CrPV accumulation and mortality (41). This antiviral effect may confer a positive selective advantage to Wolbachia-infected flies.
-
More than 18 viruses are known to infect bees (4, 7, 14), including five dicistroviruses; ABPV; Kashmir bee virus, KBV; BQCV; IAPV and an isolate of CrPV (CrPVBEE)(22). IAPV, which is closely related to KBV and ABPV but genetically and serologically distinct, was described in 2007 following severe honey bee mortality in Israeli apiculture (61). Some bees were found to harbor a segment of the IAPV genome and the presence of the virus segment correlated with resistance to IAPV infection (62). IAPV virions also contained short defective interfering (DI) -like RNAs, some of which contain host sequences, which were recognized and replicated efficiently by the viral replicase.
The presence of IAPV has been correlated with honey bee losses referred to as "Colony Collapse Disorder" (CCD) in the United States (23), with IAPV as a statistically significant marker for CCD. While IAPV as the causative agent of CCD is still under investigation, IAPV has been isolated from bees in Israel, Australia, USA and France (10, 23, 61, 74).
In addition to problems associated with the presence of honey bee viruses, infestation of hives with the varroa mite, Varroa destructor, results in suppressed immune competency, which facilitates virus replication in the honey bee (89, 90, 104, 105). Mite-induced suppression of the immune system results in activation of persistent, latent viral infection, and the mites also play a role in transmission of the honey bee viruses (90).
-
Many of the invertebrate insect hosts of dicistro-viruses are pests of various sorts: The hemipteran hosts of RhPV, ALPV, HiPV, and Plautia stali intestine virus (PSIV) are phytophagous and are all important vectors of plant viral disease. The host of TrV is a hematophagous reduviid bug and an important vector of the protozoan parasite Trypanosoma cruzi, the causal agent of Chagas disease (68). There are several examples of the use of small RNA insect viruses for pest control (19, 88) including use of CrPV for control of the olive fruit fly (60) and Helicoverpa armigera stunt virus (HaSV; Tetraviridae) for control of Helicoverpa armigera (18). Hence, the practical use of dicistroviruses for management of insect pests is the subject of ongoing research. Following is a brief description of three examples for potential use of dicistroviruses for insect pest management.
The olive fruit fly is the most serious pest of olives. CrPV replicates in adult olive fruit flies with 50% mortality within 5 days and close to 80% by 12 days after infection (59). For management of these flies, targeting the adult has potential for incorporation into integrated pest management programs using baits for virus dissemination within the fly population (58).
The red imported fire ant, Solenopsis invicta, introduced into the United States in the early 1900s has spread from North Carolina to California, thereby escaping natural enemies found in Brazil. Solenopsis invicta virus 1 (SINV-1) was one of several viruses identified during sequencing of an S. invicta expression library (95, 97). Of 167 ant nests tested, almost 23% were infected with SINV-1 with infection rates as high as 88%. All developmental stages were susceptible to infection with SINV-1 with the midgut being the primary site of infection (38). Although symptoms were not apparent, infected colonies died when transferred to the laboratory. The potential of this and other viruses isolated from S. invicta for management of the red imported fire ant is under investigation (96).
HoCV-1 was identified following the arrival of the polyphagous glassy-winged sharp shooter (GWSS), Homalodisca coagulata in California in the late 1980s (44). GWSS has a voracious appetite and vectors several plant pathogens including Xylella fastidiosa, the causative agent of Pierce's disease of grapes. Vineyards in Temecula Valley in California lost one-third of their vines over a four year period as a result of GWSS-transmitted Pierce's disease. This insect has now invaded the islands of Moorea, Easter Island, Tahiti and Oahu and presents a significant threat to agricultural and ornamental operations. Infection of GWSS with HoCV-1 is focused in the midgut and all life stages become infected (45). The impact of infection on GWSS and potential for use of HoCV-1 for management of GWSS has yet to be determined.
-
The development of the first infectious clone of a dicistrovirus will facilitate research into the fundamental biology of dicistroviruses. With such knowledge comes the potential for informed management of dicistrovirus disease of beneficial invertebrates such as honey bees and shrimp. With the ongoing agricultural problems associated with invasive insect pests, and the urgent need for development of environmentally benign pest control measures, the potential use of dicistroviruses for pest management has come under scrutiny. This need combined with the exponential increase in genomics research (95, 97), will continue to result in identification of new dicistroviruses and investigation of their potential use for pest management.














 DownLoad:
DownLoad: