-
The antiviral properties of sulfated polysaccharides were first recognised several years ago[1]. In recent years, screening assays of the antiviral activity of extracts from a number of marine algae such as Acanthophora spicifera[6], Gracilaria corticata[21], Ulva lactuca[14] has led to the identification of a number of carbohydrate polymers with potent inhibitory effects against several viruses[9, 10, 23]. These poly-saccharides include carrageenans, fucans, mannans, rhamnan sulfates and sulfated galactans[5, 17, 18, 22]. Thus, the antiviral potential of sulfated polysaccharides extracted from algae becomes of considerable interest. Although there is a lack of information about their chemical structures and physiological activities.
Gracilaria lemaneiformis, phylum rhodophyta, family Gracilariaceae, genus Gracilaria, is a important bioactive substance that is mainly cultured near the southeast coast of China. Polysaccharides in Gracilaria lemaneiformis consist of D-Galactose and 3, 6-Anhydro-L-Galactose and contain 10.80% mass percentage weight of sulfate groups[26]. Gracilaria lemaneiformis possesses various bioactive functions such as antimutagenic, antitumor, antiviral, antioxidant, anticoagulant and immunomodulation effects[3, 7]. However, no studies on the anti-influenza virus activity of the polysaccharides from Gracilaria lemaneiformis, to the best of our knowledge, have been reported.
The purpose of the present study was to isolate polysaccharide fractions from the red alga Gracilaria lemaneiformis and to investigate their antiviral activity and mechanism against human influenza virus (H1-364).
HTML
-
Samples of Gracilaria lemaneiformis were collected from the Nan-ao island of China from October to December 2008. The gathered material was sorted, washed and dried immediately by forced air circulation at 50-60℃.
-
The algal powder was extracted sequentially with: ⅰ) 100-fold volume of water at 80℃ for 4 h, repeated twice; ⅱ) After centrifugation (7 000r/min, 10min, 25℃), the supernatant was concentrated 3-fold and 95% EtOH was added to a final concentration of 80% (v/v). The deposition was collected and freeze dried; ⅲ) The crude polysaccharide was dissolved and 70% (w/v) Trichloroacetic acid (TCA) was added to a final concentration of 4%, at 4℃ overnight. After centrifugation (7 000r/min, 10min, 4℃), the supernatant was neutralized by sodium hydroxide; ⅳ) The supernatant was concentrated 3-fold and dialyzed in a DEAE cellulose bag against distilled water for 3 days. The dialyzed solution was collected and freeze dried (designated PGL).The PGL contained polysaccharides (94.48%) and protein (0.98%). The PGL was purified using a DEAE-cellulose-52 column. Three fractions were eluted by various concentrations sodium-chloride (designated GL-1, GL-2 and GL-3, respectively). GL-1, GL-2 and GL-3 showed a single symmetrical peak, respectively, on sephadex G-100 gel-chromatography columns. Thus we deduced that these were homo-geneous polysaccharides.
-
Content of polysaccharides was determined by the phenol-sulfuric acid[8]and sulfate content was determined using a modified turbidometric barium chloride method[4].
-
Solutions (3 mL) of samples in 0.05mol/L sodium-chloride were loaded to a column of (90 cm×2.6 cm) sephadex G-100 equilibrated with the same solution. The column was eluted ascendingly with the same solution at 20mL/h and the temperature was 28-30℃. Elution of polysaccharide was expressed as a function of the partition coefficient Kav[Kav= (Ve-V0) / (Vt-V0), with Vt and V0 being the total and void volume of the column determined as the elution volume of glucose and Blue dextran (2 000 Da), respectively and Ve is the elution volume of the sample]. The column was calibrated with standard dextrans within a molecular weight range of 10.0-70.0 kDa. Fractions (5mL) were collected and analysed for polysaccharides content using glucose as standard by phenol-sulfuric acid.
-
Infrared spectra of sample (KBr disc) were recorded on a FTIR Avatar 360 spectrophotometer in 400-4 000cm-1. All determinations were done at least in duplicate.
-
MDCK (Madin-Darby Canine Kidney Epithelial) Cells were used as target cells for viral infection. It grew in MEM basal medium with 10% fetal calf serum. In the antiviral assay, the MEM basal medium was supplemented with 2 μg/mL TPCK-pancreatin. The strain of human influenza virus H1-364, which was propagated in MDCK cells, was obtained from the International Infection and Immunity Institute of Shantou University. The virus was stored at -80℃ until use.
-
The virulence of H1-364 virus was expressed as median tissue culture infective dose (TCID50). H1-364 was prepared by 10-fold dilution (10-1-10-8). Then 100 μL of viral solution were added to each well of 96-well plates including MDCK cells monolayers, with four wells for each concentration. MDCK cells (added to 100 μL MEM basal medium with 2 μg/mL TPCK-pancreatin) was used as control. After 1.5 h of incubation at 37℃, 100 μL of MEM basal medium with 2 μg/mL TPCK-pancreatin was added to each well. After 4 d, the cytopathic effect was observed and recorded. The TCID50 value of H1-364 was calculated by the Reed-Muench formula.
-
MDCK cell viability was measured by the MTT method[5]. The MDCK cells monolayers, grown in 96-well plates at 37℃ with 5% CO2 for 18-24 h, were incubated to various concentrations of the different polysaccharides, with four wells for each concentration, in addition, MDCK cells (untreated and treated with various concentrations of Ribavirin Injection, namely RI) were used as negative and positive control groups, respectively. Then 100 μL of MEM basal medium with 10% fetal calf serum was added to each well. After 48 h of incubation at 37℃ with 5% CO2, the supernatant was removed and 10 μL of MEM basal medium with 10% fetal calf serum containing 5 mg/mL MTT was added to each well, and after 4 h of incubation and 1 000 r/min centrifuge, the supernatant was removed and 100μL of dimethylsulfoxide (DMSO) was added to each well. After vigorous shaking 5 min, absorbance was measured in a microplate reader at 570nm. The maximal no-cytotoxic concentration (TC0) was calculated as the concentration required to retain cell viability by 90%, and the cytotoxic concentration 50% (TC50) was calculated as the concentration necessary to reduce cell viability by 50%. Cell survival ratio (%) was calculated as Atc / Acc ×100 %. Atc and Acc denote the absorbance of the test substance with MDCK cells and absorbance of cell control, respectively.
-
To investigate the antiviral activity of different polysaccharides, 100 μL of 100 TCID50 H1-364 per well was absorbed by monolayers of MDCK cells for 1.5 h. Various concentrations of the different poly-saccharides were added to cultured cells infected with 100 TCID50 H1-364 at 37℃ with 5% CO2 for 48 h, with four wells for each concentration. The MDCK cells and virus were as negative and positive control groups, respectively. After 48 h of incubation at 37℃ with 5% CO2, the MTT test was carried out as previously described. Viral inhibition ratio (%) was calculated as (Atv-Acv)/(Acd-Acv)×100%. Atv indicates the absorbance of the test substance with viral infected cells. Acv and Acd denote the absorbance of the virus control and absorbance of the cell control, respectively.
-
The 100 μL of various concentrations of PGL and 100 μL of initial virus solution was confluently cultured in 96-well plates at 37℃ for 1 h. Each tube containing H1-364 were 10-fold series diluted (10-1-10-8). The TCID50 value of H1-364 was calculated by the Reed-Muench formula[11].
-
Antiviral activity was evaluated by a virus plaque forming unit assay[12]. MDCK cells monolayers grown in 24-well plates were incubated to various concen-trations of PGL at 37℃ with 5% CO2, with 200 μL for each concentration per well. After 3 h, the supernatant was removed and the cells were infected with 100 TCID50 H1-364 per well. After 2 h of adsorption at 37℃, the supernatant including virus was collected and stored at -70℃. The supernatant was tested by Plaque assay. Titer of virus in collected supernatant was calculated as follows: PFU/mL=average plaque forming unit (PFU)/dilution ratio × inoculation quantity (mL). All determinations were performed twice and each in duplicate.
-
Antiviral activity of PGL against virus was evaluated by MTT method as previously described. MDCK cells monolayers grown in 24-well plates were incubated to various concentrations of PGL when infected with 100 TCID50 H1-364. After 2 h, un-adsorbed viruses and PGL were removed and 100 μL of MEM basal medium with 10% fetal calf serum was added to each well. After 48-72 h of incubation at 37℃, CPE was observed under microscope. The MTT test was carried out as previously described. Viral inhibition ratio (%) was calculated as (Atv-Acv) / (Acd-Acv) × 100%. Atv indicates the absorbance of the test substance with viral infected cells. Acv and Acd denote the absorbance of the virus control and absorbance of the cell control, respectively.
-
MDCK cells in 24-well plates were infected with 100 TCID50 H1-364 at 37℃ for 2 h and then treated with various concentrations of PGL for 24-72 h. The cytopathic effect was observed under microscope. The MTT test and viral inhibition ratio determined as above
-
MDCK cells infected with H1-364 in 24-well plates were incubated to different concentrations of PGL, with three wells for each concentration and MDCK cells infected with H1-364 in 24-well plates without adding PGL was used as a control group. When 75% of cells in the control group showed CPE at 37℃ with 5% CO2, cell culture was terminated, and cell culture supernatant and MDCK cells were stored at -70℃ and then were tested by Plaque assay. (MDCK cells needed to be melted three times). The virus plaque forming unit test was carried out as previously described. Virus titer was determined as described above.
-
To determine the TC50(cytotoxic concentration 50%) and IC50(inhibitory concentration 50%) of different polysaccharides fractions from Gracilaria lemaneiformis, data were analyzed using Probit regression for the statistical package "SPSS 12.0 for Windows".
Plant material
Isolation and purification of polysaccharides
Sugars and sulfate estimation
Molecular weight
Infra red spectroscopy
Cell lines and viruses
Virulence of H1-364 assay
Cytotoxicity assay
Antiviral assay
Interaction of PGL and H1-364 virus previously
Target cells treated with PGL previously
Interaction of PGL, virus and cells
Viral replication assay
Viral release assay
Statistics analysis
-
A hot water-extracted polysaccharide (designated PGL) was obtained from algal powder of Gracilaria lemaneiformis. Using a DEAE-cellulose-52 column, three fractions were eluted by various concentrations of sodium-chloride (designated GL-1, GL-2 and GL-3, respectively). GL-1, GL-2 and GL-3 showed a single symmetrical peak, respectively, on sephadex G-100 gel-chromatography columns (data not shown). Thus we deduced that these were homogeneous poly-saccharides. In addition, the characteristics of the different eluted fractions were given in Table 1.

Table 1. Properties of different polysaccharides
-
The FTIR Spectra of the samples (PGL, PG-1, PG-2 and PG-3 respectively from the top-down) are shown in Fig. 1. These samples exhibit absorption peaks at 3400 cm-1, 2933 cm-1 and 1080 cm-1, which are characteristic absorptions of -OH, C-H and C-O, respectively[25]. The IR absorption of at 931cm-1 is the characteristic absorption of 3, 6-Anhydro-L-galactose. Infrared spectroscopy provides useful information for the position of sulfate groups of polysaccharides. The IR spectra shows an absorption band at 1249 cm-1, indicating the presence of the total sulfate ester[16]. From distilled water eluted fractions (GL-1) to 1.0 mol/L NaCl eluted fractions (GL-2, GL-3) the 1249cm-1absorption peak intensity gradually increases in the order GL-1/GL-2/GL-3, indicating the sulfate content of eluted fractions increased with the polarity of the eluating solution. A small absorption peak at 810 cm-1 indicates that there is a sulfate at galactose-C2[19], but, the IR spectrum of GL-1 does not show an obvious absorption peak at 810 cm-1. The FTIR Spectra of the samples suggest that these polysaccharide samples are sulfated polysaccharides, but the GL-1 fraction appears to contain relatively little sulfate groups, thus the results of the analysis of IR spectra is consistent with the the estimates of sulfate content in polysaccharide samples.
-
We first evaluated the cytotoxicity of different polysaccharides against the target cells for antiviral assay by the MTT method. The TC0 values of GL-1 and RI were 250.00 μg/mL, the TC0 values of the others were 125.00 μg/mL. In addition, when the polysaccharides concentration was greater than 250μg/ mL then the greater the number of sulfate groups, the lower the cell viability (Table 1 and Table 2). The mass percentage content of sulfate groups of different polysaccharides is shown in Table 1. These results imply that MDCK cytotoxicity was caused by the sulfate groups of the polysaccharides.

Table 2. Effect of different polysaccharides and RI on survival of MDCK cells
-
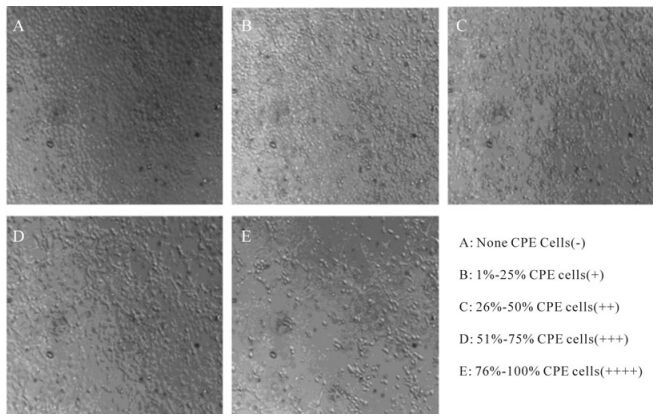
The morphological effects of H1-364 virus on MDCK cells is shown in Fig. 2. Cytopathic changes of MDCK cells include rounded cells, cellular atrophy, cellular breakage and exfoliated cells. The TCID50 value of H1-364 infection was 10-5.
-
A summary of the antiviral activities of different polysaccharides is shown in Table 3 and Table 4. When the TC0 values of PGL and GL-2 were 125.00 μg/mL, the antiviral activities against H1-364 were more effective than others. In addition, our study showed that the antiviral activities were associated with content of sulfate groups in samples. When the content by mass was about 13%, the antiviral activities of polysaccharides showed more efficacy. Given the lack of toxicity for PGL cultured cells from Gracilaria lemaneiformis and high values of TI (ratio between cytotoxic TC50 and antiviral IC50) against H1-364, they were used to further investigate the drug effects on virus infection and characterize the mechanism of action.

Table 3. Inhibition ratio of different polysaccharides to H1-364.

Table 4. Effect of different polysaccharides and RI on Inhibition ratio, TC50, IC50 and TI of H1-364 virus.
-
In order to explore the anti-H1-364 action of PGL, the ability to directly inactivate virus (virucidal activity) was tested. Results showed that the TCID50 values were not significantly affected by PGL concentration. Suggesting that PGL did not reduce the virulence of H1-364 virus. Therefore PGL could not directly inactivate H1-364. Viral adsorption ability was also investigated through pre-treatment of target cells with various concentrations of PGL. Results showed that virus residue amount in the treatment groups was significantly higher than that in the control group. That is viral adsorption ability was significantly decreased through pre-treatment of target cells with various concentrations of PGL. In addition, interaction of PGL, virus and cells on viral absorption was studied by various concentrations of PGL. Results showed that the cytopathic effect was reduced when treated with various concentrations of PGL, and maximum ratio of inhibition was 83.51% when concentration was 62.50 μg/mL (Table 5).

Table 5. Effect of PGL from Gracilaria lemaneiformis on viral adsorption.
-
In order to investigate the mechanism on how PGL inhibits the infection of H1-364, effect of viral replication was studied by various concentrations of PGL (Table 6). Results showed that PGL at concentrations 125.00, 62.50, 31.25 and 15.62 μg/mL exhibited a significant effect on viral replication against H1-364 infection, and regression analyses indicated that the dependence of the antiviral effect of PGL on their concentration agreed with a Gaussian model with one unknown parameter as follows:
${Y_{_{{\rm{Inhibition ratio}}}}} = {\rm{87}}.{\rm{3}}{{\rm{e}}^{^{ - {{(\frac{{x - 86.42}}{{124.4}})}^2}}}}$ (r2= 0.9562, x means concentration of PGL). In addition, the H1-364 induced cytopathic effect was reduced when treated with PGL.
Table 6. Effect of PGL from Gracilaria lemaneiformis on viral replication.
-
Effect on H1-364 viral release was studied by various concentrations of PGL (Table 7). Two experimental results showed that the ratio of extracellular virus amount to total is not significant difference. It is implied that H1-364 viral release is not affected by PGL.

Table 7. Effect of PGL from Gracilaria lemaneiformis on viral release (PFU/mL).
Isolation, purification and character of poly-saccharide from Gracilaria lemaneiformis
IR spectroscopy
Cytotoxicity
H1-364 induced cytopathic effect
Antiviral activities
Effect of PGL on viral adsorption
Effect of PGL on viral replication
Effect of PGL on viral release
-
Influenza virus could cause an infection of the respiratory tract and lead to more serious illness than common cold. Amantadine, rimantadine, zanamivir and oseltamivir are four currently licensed agents in the United States. Amantadine and rimantadine are used for the prevention and treatment of influenza A infection. Zanamivir and oseltamivir could exhibit the activity of anti-influenza A and B viruses as neuraminidase inhibitors. But these antiviral drugs would cause side effects on central nervous system and gastrointestinal tract, and induce easily the emergence of drug-resistant viruses[15]. Therefore, it is necessary for screening new drug of the antiviral activity.
Our study showed anti-influenza virus activity of sulfated polysaccharide fractions from Gracilaria lemaneiformis, and these activities were associated with content of sulfate groups in polysaccharide fractions. Due to the abundance of PGL from Gracilaria lemaneiformis, further comprehensive investigation should be carried out as a new drug of the anti-influenza virus activity.
Different investigators have reported that the antiviral activity of sulfated polysaccharides increases with the degree of sulfation and the molecular weight of the macromolecule [13, 20]. However, our studies showed that the antiviral activities were also associated with content of sulfate groups in polysaccharide fractions. When the mass content was about 13%, the antiviral activities of polysaccharides (PGL and GL-2) were higher. Conversely, the antiviral activities of polysaccharides (GL-1 0.45%and GL-322.16%) was decreased, This is consistent with observations that have found that when the molecule contain 1.5-2.0 sulfate groups per sugar residue, sulfated poly-saccharides exhibit higher antiviral activity[27]. In the present studies, PGL could not directly inactivate H1-364, and viral release was also not affected, but viral adsorption and replication abilities were significantly decreased. This is possibly caused by characteristics of the polyanions. On one hand, viral adsorption on target cells was interdicted by the combination of polyanions and virus, thereby reverse transcripatase activity was inhibited, and on the other hand, viral adsorption was disturbed by the combination of polyanions and positive charges of cellular surface [24]. In addition, the cytotoxicities of sulfated polysaccharide to MDCK cells were associated with sulfate groups content in poly-saccharide fractions, and the greater the number of sulfate groups, the lower the cell viability. Thus, a possible explanation is that cellular physiological functions were affected by the combination of polyanions and positive charges on the cellular surface.
H3N2, a seasonal strain of influenza, has popped up in many East Asian countries now, and some variants in circulation may outfox the seasonal vaccine in use. It is ceitainly necessary to screen new drug of the antiviral activity for H3N2.But there was only H1-364 virus and no H3N2 strain in International Infection and Immunity Institute of Shantou University at that time, we just used H1-364 virus in this study. It is the weakness of our study. Because of the great diversity in chemical structure among sulfated polysaccharides with antiviral activity, the structure-activity relationship for this class of polyanions has not been clearly established which will be our future work. An animal model is also needed to further evaluate effects and will be carried out in the future studies. Additionally, we plan to use HA cells instead of CPE, incorporate additional controls such as H3N2 strain and evaluate viral replication using real time RT-PCR.















 DownLoad:
DownLoad: