-
Classical swine fever (CSF) is a highly contagious disease of pigs, caused by infection with classical swine fever virus (CSFV), leading to significant economic losses worldwide[7, 17, 18, 24]. CSF disease control is primarily dependent on preventive vaccination and the conventional attenuated live C-strain vaccine is considered as one of the most effective preventive vaccines for the control of CSF and has been widely used in many countries, including China.
CSFV, a member of the genus Pestivirus within the family Flaviviridae, contains a genome of approximately 12.5kb with a single large open reading frame (ORF). The ORF is translated to a polyprotein that is processed into 12 mature proteins by viral and host cell proteases[19, 27]. Among the CSFV proteins, the structural proteins Erns, E2, and the nonstructural protein NS3 are the three immunogens inducing detectable antibodies[12, 22, 23]. Specific anti-E2 is a major virus neutralizing antibody[9, 11, 29] whereas antibodies against Erns and NS3 have only low neutralizing activity, or none at all[11, 31].
Detection of specific antibodies is widely used for diagnosis and surveillance of viral diseases. The conventional methods for the detection of antibodies to CSFV are the virus neutralization assay and the enzyme-linked immunosorbent assay (ELISA)[5, 21, 26]. Regarded as a gold standard, the virus neutralization assay nevertheless has several limitations including a time-consuming, the requirement of cell culture, the need for live virus manipulation, and expensive and hampered standardization due to subjective plaque-reading. Furthermore, ELISA based on viral antigens of CSFV is disadvantageous because of low virus titer in tissue cultures and the complicated viral antigen preparation. Therefore, the ELISA based on recombinant E2 or Erns has been developed[3, 4, 13, 20, 22]. In the present study, multiple ELISAs based on the recombinant CSFV Erns, the truncated E2 (tE2) and NS3 were developed. Anti-Erns, anti-E2 and anti-NS3 antibody titers in sera of vaccinated pigs were then evaluated by the multiple ELISAs.
HTML
-
The serum panel consisted of vaccinated (n = 201) and unvaccinated pig sera (n = 64). Positive sera of pigs vaccinated with commercial attenuated vaccines (the Chinese lapinized vaccine, C-strain) from 6 farms were kindly supplied by the Wuhan Center for Animal Disease Control. Positive serum samples in pigs vaccinated with an attenuated C-strain vaccine were collected at a 1, 3 or 5 month intervals post vaccination (p.v.), respectively. Negative sera were collected from 6-week-old unvaccinated piglets in 2 CSFV-free farms. Neutralization antibody titers in pig sera were tested by the virus neutralization assay.
-
To obtain the recombinant antigens, the full-length (fl)Erns (from position 1175 to 1855), the truncated E2 (tE2) (from 2441 to 2972nt), and flNS3 (from 5141 to 7189) gene fragments were amplified from a full-length infectious cDNA clone (pT7SM) of CSFV Shimen Strain[32]. Oligonucleotides employed to amplify Erns, tE2 and NS3 fragments are listed in table 1. Erns and NS3 fragments were cloned into pET-28α vectors (Novagen) with BamH Ⅰ and EcoR Ⅰ or with Bgl Ⅱ and Sal Ⅰ, to generate pET/Erns and pET/NS3, respectively. Similarly, tE2 was cloned into pMAL-p2x (New England Biolabs) with BamH Ⅰ and Hind Ⅲ, to generate pMAL/tE2. All clones were confirmed by DNA sequencing.

Table 1. Oligonucleotides employed to amplify Erns, tE2 and NS3 coding regions
To express and purify Erns, tE2 or NS3 protein, the recombinant plasmid pET/Erns, pMAL/tE2 or pET/NS3 was transformed in competent E. coli BL21 (DE3) CodonPlus cells (Stratagene, San Diego, CA). The E. coli cells harbouring pET/Erns, pMAL/tE2 or pET/NS3 were grown at 37 ℃ in LB medium containing antibiotics to an optical density of 0.6 at 600 nm with shaking. Isopropyl-1-thio-b-D-galactopyranoside (IPTG) (Sigma-Aldrich, St Louis, Missouri, USA) was added to a final concentration of 0.5 mmol/L and the bacterial cells were grown for five additional hours at 30 ℃, pelleted by centrifugation. The cells were resuspended in binding buffer (20 mmol/L imidazole, 500 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.4 for Erns and NS3 purification or 10 mmol/L HEPES-KOH, 200 mmol/L NaCl, 1 mmol/L M EDTA, pH 7.4 for tE2 purification) containing 1 mmol/L PMSF and lysozyme (100 μg/mL), incubated on ice for 30 min, followed by sonication. Cell debris was removed by centrifugation at 12, 000 r/min for 30 min. For Erns and NS3 purification, the supernatant was added to the Ni-NTA agarose resin column (Amersham, USA). After incubation and washing, the Erns or NS3 was eluted with elution buffer (500 mmol/L imidazole, 500 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.4). For tE2 purification, the supernatant was added into a column of amylose resin (Biolabs, New England). After washing, the tE2 was eluted with elution buffer (10 mmol/L HEPES-KOH, 200 mmol/L NaCl, 1 mmol/L EDTA, 10 mmol/L maltose, pH 7.4). Peak fractions were pooled and then dialyzed against a buffer containing 50 mmol/L Tris-HCl (pH 7.6), 150 mmol/L NaCl, and 20% glycerol. The purified proteins were analyzed by SDS-PAGE and Western blot. After concentration as previously described[32], the proteins were divided into aliquots and stored at -80 ℃.
-
Independent ELISAs based on the recombinant Erns, tE2 or NS3 antigen were developed. Optimal concentration of each antigen as well as pig sera and anti-pig IgG conjugated with horseradish peroxidase (HRP) were determined by checkerboard titration.
96-well microtiter plates (Greiner, Germany) were coated with an optimal concentration of the purified antigens (0.2 μg/well for Ernsand tE2; 0.3 μg /well for NS3). The coating antigens in 0.06 mmol/L carbonate buffer (pH9.6) were aliquoted into each well (100 μL/well) and incubated overnight (18-20 h) at 4 ℃. After washing with phosphate-buffered saline containing 0.05% Tween-20 (PBST), the plates were blocked for 1 h at 37 ℃ with 100 µL/well of PBST containing 3% BSA (pH7.4). After several washes, 100 μL of diluted pig sera (1:200) in PBST was added to each well in duplicate and incubated for 1 h at 37 ℃. After washing, the plates were incubated for 1 h at 37 ℃ with 100 μL/well of HRP-labeled anti-pig IgG (Sigma-Aldrich, St. Louis, MO, USA) diluted 1:5000 in PBST. Following several washes, plates were incubated for 10-30 min with 0.1 mL of 0.045% H2O2 and 0.4 mg/mL of O-phenylenediamine dihydrochloride (OPD) (Sigma-Aldrich) in phosphate-citrate buffer (0.1 mol/L citric acid, 0.2 mol/L sodium phosphate dibasic, pH 5.0). The reaction was stopped by adding 100 μL of 2 mmol/L H2SO4 and the absorbance was measured at 492 nm using an ELISA reader (Multiskan MK3, Thermo, USA).
-
All pig sera employed in these assays were tested for the presence of virus neutralizing antibodies against CSFV as previously described[30]. Briefly, 2-fold serially diluted sera were incubated with equal volumes of 100 TCID50 CSFV (Shimen strain) in a 96-well plate for 1 h and then the virus/serum mixture was replaced with fresh medium. After incubation in a humidified atmosphere containing 5% CO2 for 3 days, the confluent monolayer was fixed with 50% methanol and 50% acetone at -20 ℃ for 1 h and extensively washed with PBST. A rabbit polyclonal anti-CSFV serum (1:500 dilution) was added and incubated for 1 h at 37 ℃. Plates were washed and an FITC-conjugated goat anti-rabbit IgG (1:200 dilution) was added. The serum neutralization titers were recorded as the reciprocal of the highest serum dilution at which the fluorescence was 50% or less than that of the virus infected control well.
-
The cut-off value of each ELISA was determined by receiver operating characteristic (ROC) curve with default settings (software SPSS, version 15.0). Optical density (OD) values at 492 nm from a total of 201 pig sera of vaccinated pigs and 64 negative sera of unvaccinated piglets were analyzed by the ROC curves. Optimal cut-off values were determined by ROC analysis by comparing false negative (FN) and false positive (FP) rates for every possible cut-off. ROC curves were created by plotting the sensitivity (Sensitivity = TP / (TP + FN)) against 1-specificity (Specificity = TN / (FP + TN)) for different cut-off values of each ELISA. The intra-and inter-assay variation coefficients (VC) were estimated as previously described[2, 10].
Test sera
Cloning, expression and purification of the recombinant Erns, tE2 and NS3 proteins
Indirect ELISA
Virus neutralization assay
Determination of cut-off values and variation coefficients
-
The CSFV Erns, tE2 or NS3 was separately expressed in E. coli and purified by affinity chromatography. SDS-PAGE analysis showed that the recombinant Erns, tE2 and NS3 achieved high-purify and their sizes were 28 kDa, 62 kDa and 80 kDa, respectively (Fig. 1A). Western blotting identified that these proteins were reactive with specific anti-E2 monoclonal or anti-CSFV rabbit polyclonal antibody (Fig. 1B). The purified Erns, tE2 and NS3 were subsequently used as coating antigens for the ELISAs.
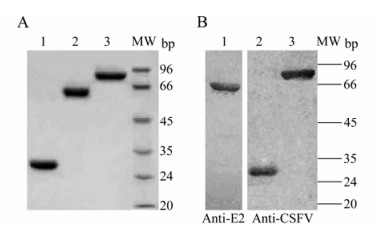
Figure 1. Purification and analysis of the recombinant Erns, tE2 and NS3 of CSFV in E. coli. A: The purified Erns (lane 1), tE2-MBP (lane 2) and NS3 (lane 3) proteins were analyzed by SDS-PAGE. B: Western blot analysis of the tE2-MBP (lane 1), Erns (lane 2) and NS3 (lane 3) proteins expressed in E. coli using anti-E2 monoclonal antibody or rabbit anti-CSFV polyclonal antibodies. MW, standards of molecular weight.
-
The results of checkerboard titration showed that the coating antigens at 0.2 μg/well for Erns and tE2 proteins, or at 0.3 μg /well for NS3 gave the highest ratio OD492 values for positive sera to negative sera (P/N). Thus, the optimal coating antigens were set at 0.2 μg/well for Erns and tE2, or 0.3 μg /well for NS3. The optimal dilution of pig sera and anti-pig IgG was 1:200 and 1 : 5000, respectively. In addition, PBST containing 1% BSA for serum dilution and 3% BSA for blocking gave the better P/N values.
-
Once the final procedures were set up, the specificity and sensitivity of multiple ELISAs were evaluated through 201 positive sera of vaccinated pigs and 64 negative sera of unvaccinated piglets. The cut-off values were optimized by the ROC analyses of software SPSS (Version 15.0) by non-parametric methods at 95% accuracy level; areas under the ROC curves corresponding to the Erns, tE2 and NS3 were all more than 0.98. The values that achieved the best relationship between diagnostic sensitivity and specificity were selected as the cut-off value of each ELISA. The corresponding sensitivities to Erns, tE2 and NS3 were 96%, 98.5% and 96% while the specificities were 95.3%, 93.7% and 93.7%, respectively (Table. 2).

Table 2. Sensitivity (Se) and specificity (Sp) values estimated for the ELISA tests based on Erns, tE2 and NS3 recombinant antigens using ROC curve by SPSS version 15.0
When all the parameters of each ELISA were established, the assays developed were applied to the testing of 201 positive serum samples of vaccinated pigs and 64 negative serum samples of unvaccinated piglets, previously characterized by the virus neutralization assay. The three assays showed high levels of sensitivity and specificity when compared with the reference test. ELISA-E2 data showed that 4 out of 64 serum samples of unvaccinated piglets were positive and 3 out of 201 positive samples of vaccinated pigs were negative (Fig. 2B). For the ELISA-Erns, 3 out of 64 negative samples showed ELISA-Erns values above the established cut-off point and 8 out of 201 positive samples showed values below that point (Fig. 2A). Similarly, for ELISA-NS3 with a cut-off value set to 0.167, 4 out of 64 negative samples showed higher and 8 out of 201 serum samples of vaccinated pigs were lower than the established cut-off point (Fig. 2C). Virus neutralization assay demonstrated that no CSFV neutralizing antibodies in sera of all unvaccinated piglets were detected. The CSFV neutralizing antibodies were positive in sera of all vaccinated pigs. The variation analysis indicated that both intra-and inter-assay VCs were lower than 15% for raw absorbance values (Table. 3), suggesting adequate reproducibility and repeatability of the ELISAs.

Table 3. Intra-and Inter-assay variation coefficient (CV) estimated for the ELISA tests based on Erns, tE2 and NS3 recombinant antigens
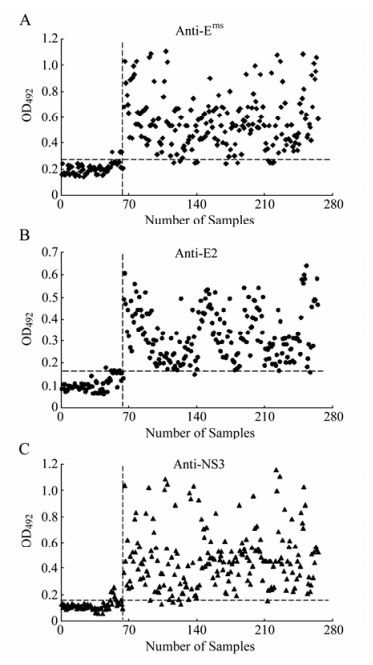
Figure 2. The multiple ELISAs developed with the recombinant Erns (A), tE2 (B) or NS3 (C) antigen. A total of 64 negative serum samples and 201 positive serum samples, which were characterized by virus neutralization assay, were applied to each ELISA. Each sample was tested in duplicate and the corresponding result was expressed as the OD492nm value obtained with the recombinant antigen (Erns, tE2 or NS3). The optimum cut-off value determined by ROC analysis is indicated by a broken horizontal line.
-
To investigate Erns, E2 and NS3 antibody responses in pigs, specific antibodies in 90 sera of vaccinated pigs were measured separately at 1, 3 and 5 months p.v. The data showed that a single dose of vaccination induced the specific anti-Erns, anti-E2 and anti-NS3 responses in sera of pigs at 1st month p.v. and there was no significant difference of antibody titers between the 1st and 5th month p.v. (Fig. 3). The results demonstrated that a single vaccination with C-strain can confer anti-CSFV humoral responses lasting at least 5 months, which further confirmed the results previously reported[28].
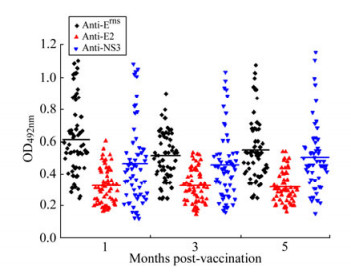
Figure 3. Antibody responses of vaccinated pigs to Erns, E2 and NS3 detected by the multiple ELISAs. Ninety serum samples from vaccinated pigs at 1, 3, or 5 months p.v. were analyzed for reactivity with immobilized Erns, tE2 and NS3, using the optimized conditions as described in the text. Mean OD492nm values are represented as a solid line.
Purification and identification of recombinant Erns, tE2 and NS3 proteins
Development of multiple ELISAs based on the Erns, tE2 and NS3 antigens
Validation of the ELISA-Erns, ELISA-E2 and ELISA-NS3
Erns, E2 and NS3 antibody responses in pigs vaccinated with the C-strain
-
Antibodies against Erns, E2 and NS3 are measured in sera of pigs are common indicators of infection with CSFV. Detection of specific Erns or E2 antibodies has widely been used for diagnosis and surveillance of CSF diseases. Erns, E2 and NS3 of Pestiviruses have been expressed in baculovirus/insect cells or E. coli, and used as diagnostic antigens[6, 13, 29]. ELISA based on the recombinant Erns, E2 or chimeric Erns/E2 antigen has been developed for detection of CSFV infection[4, 12, 14]. In BVDV, multiple ELISAs based on recombinant Erns, E2 and NS3 antigens have been developed and applied to determine the pattern of anti-BVDV antibodies in bovine serum samples[2]. The E2 subunit or inactivated BVDV vaccine exhibits properties of the "marker vaccines" when an appropriated ELISA based on NS3 is applied[1, 16]. However, in CSFV, development of ELISA-NS3 or multiple ELISAs have not been reported. Our previous results demonstrated that the CSFV Erns and NS3 expressed in eukaryotic or prokaryotic expression system revealed bioactivity[15, 32]. In this study the Erns, tE2 and NS3 proteins of CSFV were expressed from E. coli. Western blotting indicated that three proteins expressed from E. coli could be reactive with anti-E2 monoclonal antibody and anti-CSFV polyclonal antibody. Because of hydrophobic amino acids and rare codons, the full-length E2 has a lower expression level in E.coli. By removing the C-terminal domain and fusing the N-terminal part of E2 with maltose-binding protein (MBP), a high expression level of the tE2 was obtained in strain BL21 CondonPlus (DE3)-RIL. Because the proteins were expressed mainly in the form of inclusion bodies, we also purified the recombinant Erns, tE2 and NS3 by SDS-PAGE separation and gel recovery. Western blotting confirmed that the purified proteins exhibited reactivity with specific anti-CSFV antibody (data not shown), which could be a simple, useful method for laboratory preparation of recombinant protein antigens.
Different methods were applied to determine the optimal cut-off value for ELISA. The negative mean plus three standard deviations (Xneg+3σ) is the traditional cut-off value[25]. In the Xneg+3σ determined point, the cut-off values corresponding to ELISA-Erns, ELISA-E2 and ELISA-NS3 are 0.301, 0.186 and 0.183, respectively. When the cut-off values are considered, the sensitivities of the ELISA-Erns, ELISA-E2 and ELISA-NS3 are 95%, 94.5% and 95.5%, while the specificities are 95.3%, 95.3% and 93.7%, respectively. In the multiple ELISAs described, the cut-off values were determined by ROC analysis. Based on the cut-off values established from these two methods, the multiple ELISAs are specific, sensitive and reproducible. Multiple ELISA data revealed that several negative serum samples of unvaccinated piglets showed values above the cut-off point and that some positive serum samples of vaccinated pigs exhibited values below the cut-off point. For the former, a possible explanation is that the multiple ELISAs are sensitive enough to test the low levels of maternal antibodies. For the latter, it is could be due to low serum neutralization activity plus subjective plaque-reading or to deviation in the ELISA. By Western blotting, the reactivity of positive sera measured by ELISAs with the recombinant Erns, E2 and NS3 proteins were further confirmed and their consistency was observed. These results suggested the multiple ELISAs developed were effective and accurate for detection of specific antibodies in pig sera. Furthermore, the ELISA-Erns and ELISA-NS3 should be of particular interest since they could be used to detect the presence of specific antibodies (directed to Erns and NS3) that were not detectable by the virus neutralization assay with CSFV. Thus, the inclusion of Erns and NS3 would extend the range of antibodies that can be detected in comparison with E2 alone.
To evaluate the pattern of anti-CSFV antibodies p.v. with C-strain vaccine, specific anti-Erns, anti-E2 or anti-NS3 antibody titers in sera of vaccinated pigs were tested by the multiple ELISAs. Data showed that there is no significant difference of the antibody titers in sera of vaccinated pigs between 1st and 5th month p.v. with a single vaccination. However, the OD492 values of ELISA-E2 were lower in comparison with that of ELISA-Erns and ELISA-NS3. A possible explanation is that the truncated E2 protein from the prokaryotic expression system was employed as an ELISA coating antigen. Currently, a blocking ELISA based on the recombinant truncated E2 of BVDV has been developed. Importantly, the blocking ELISA measures avidity of BVDV-specific immunoglobulins as an alternative to the classic virus neutralization assay and can discriminate between infected and vaccinated animals[8]. The multiple ELISAs described here do not require the manipulation of infectious material, and simultaneous application of the three assays allows the detection of anti-CSFV antibodies in pig sera by a simple, rapid and secure methodology, so that the assays represent a valuable tool for the detection of CSFV infection and serological survey in CSFV-free countries or for the evaluation of the efficacies of live attenuated CSFV vaccines.














 DownLoad:
DownLoad: