-
The nontoxic B subunit of Shiga toxin (STxB) from Shigella dysenteriae (O'Brien A D, et al., 1992; Shaw C A, et al., 2003) is responsible for toxin binding and internalization into target cells by interacting with glycosphingolipid globotriaosyl ceramide (Gb3 or CD77) (Haicheur N, et al., 2003; Janssen K P, et al., 2006; LaCasse E C, et al., 1996). Gb3 is almost exclusively expressed in cancer cells, dendritic cells (DC) and B cells. So it is tempting to propose the use of STxB for activation of the MHC class Ⅰ-restricted E7-specific CTLs which have been shown to represent an important component of the protective and therapeutic immune response to tumors (Haicheur N, et al., 2000; Haicheur N, et al., 2003; Janssen K P, et al., 2006; Vingert B, et al., 2006). Genetic fusions of STxB with defined viral/tumor antigens have previously been incorporated in recombinant therapeutic vaccines (Choi N W, et al., 2005; Haicheur N, et al., 2000; Lee R S, et al., 1998; Nakagawa I, et al., 1999; Ohmura M, et al., 2005; Vingert B, et al., 2006). Due to the presence of certain barriers in obtaining a functionally soluble form of the recombinant STxB, scientists tend to combine STxB with vaccine candidates rather than use their genetically fused forms. However, in most cases, such peptides failed to elicit efficient immune and clinical responses (Goncalves A N, et al., 2012).
Bacteria can express the recombinant protein in insoluble or soluble forms. The former is expressed in the cytoplasm as an inclusion body with high-level protein production. (Lee R S, et al., 1998), although this high yield of heterologous protein expression may be nonfunctional and hence the tumor targeting ability of STxB will not be fulfilled (Buchner J, et al., 1992). Furthermore, due to the exposure of hydrophobic peptide regions, proteins are mainly aggregated (Sedighzadeh S S, et al., 2012; Weikert C, et al., 1998; Zhang S, et al., 2009).
In previous studies, in order to obtain soluble proteins from E. coli, several strategies have been applied, e.g. optimization of medium formulation and parameters of the cultivation process (Baneyx F, et al., 2004; Choi J H, et al., 2004; Li J, et al., 2009), application of fusion tags, coexpression of chaperones (Weikert C, et al., 1998), secretion of recombinant proteins into the periplasmic space and also modification of culture medium to incorporate diverse helper proteins and additives (Choi J H, et al., 2004).
In this study, we chose Human papillomavirus 16 (HPV16) E7 oncogene as a model antigen for a protein-based therapeutic vaccine against cervical cancer. Previous studies have showed that the immunization with HPV16 E7 protein in fusion form with certain immunoadjuvants is able to elicit specific protective immunity against TC-1 tumor growth (Lee R S, et al., 1998; Li Y L, et al., 2011). However, to date, there has not been any report about development of a protein vaccine using the whole HPV16 E7 protein fused to STxB.
In this work HPV16 E7 antigen was fused with STxB to function as a bacterial drug delivery system. Then various strategies including the use of different host strains, reduction of cultivation temperature, different concentrations of IPTG and different additives (Glycin, Triton X-100, ZnCl2), were used in order to obtain high yield of secreted recombinant protein from E. coli. We also investigated the effect of these parameters for export of recombinant protein to the growth medium. Finally, we developed a new merged strategy, involving less expensive and fewer steps, to obtain high-yield protein expression. The results of this study will facilitate achieving a functional soluble form of STxB-based vaccines.
HTML
-
Pfu and taq DNA polymerase (2.5 U/μL, Fermentas, Lithuania), Enzymes Nde Ⅰ, Sal Ⅰ and Not Ⅰ (Fermentas, Lithuania.), IPTG (Vivantis, Malaysia), Vector pET-28a(+) (Novagen USA), Vector pGEM-T (Promega, USA), and the stxB gene from Shigella dysenteriae type Ⅰ was prepared (At Imam Hossein University, Tehran, Iran) according to the method we previously described Sadraeian M, et al., 2011), and was used as the PCR template. E. coli DH5α and E. coli BL21 (DE3) were used for cloning and expression experiments. Plasmid pGEM-T Vector and pET-28a(+) were used as cloning and expression vectors respectively.
-
First step: The DNA sequence of the E7 gene was obtained from the GenBank database (accession no. AF486352) and confirmed by sequencing. Optimization of the rare codons of the E7 gene was performed using the JCAT (Java Codon Adaptation Tool) software to increase the soluble expression of E7 protein in the host E. coli BL21 (DE3). Synthetic codon optimized HPV16 E7 was cloned into pGH Vector purchased from Nedayefan Co, Iran. In this case, only the forward primer of HPV16 E7 with an Nde Ⅰ restriction site as well as signal sequence at 5' end was designed; Forward primer (HPV E7): CATATGATGAAGAAGACCGCAATCGCAATC Second step: The stxB gene (207 bp) from Shigella dysenteriae type Ⅰ was amplified from plasmid by PCR. The following oligonucleotides with Sal Ⅰ, BamH Ⅰ and Not Ⅰ restriction sites were used: Forward primer(stxB): 5′GGATCCGGCGGCGGCGGCTCTGTCGACACGCCTGATTGTGTAACTG3′. Reverse primer (stxB): ′GCGGCCGCCTAGTGGTGGTGGTGGTGGTGGTGCTCGAGTATTATCAACGAAAAATAACTTC3′. GGCGGCGGCGGCTCT is the gene codon of a nonfurin linker that is rich in glycine and serine amino acids. Here restriction sites are underlined. The stxB gene was amplified with PCR by pfu enzyme.
Third step: The PCR product of stxB was tailed with dATP, separately ligated with pGEM-E7 vector which was digested with Nde Ⅰ and BamH Ⅰ, and transformed into E. coli DH5α. Construction of pGEM E7-Linker-stxB was subsequently tested by PCR as a control test and endonuclease digestion. The insert was verified by DNA sequencing.
The reverse primer contained a 6×His tag at the C-terminus of the recombinant protein for the convenience of purification and Western blot detection of the recombinant protein.
-
The E7-stxB fragment was produced by digestion of pGEM E7-stxB by NdeⅠ and NotⅠ restriction endonuclease and was subcloned into undigested pET28a(+) as an expression vector to construct pET28a(+)-E7-stxB. Afterward pET-28a(+)-E7-stxB was confirmed by PCR and restriction enzyme digestion.
The recombinant plasmid was amplified in competent E. coli DH5α and then extracted by the alkaline lysis method (Sedighzadeh S S, et al., 2012) and stored at -20℃. The resulting plasmid was transformed via electroporation into the competent final host, E. coli BL21 (DE3), then kanamycin resistance as well as restriction enzyme digestion were used for selection and confirmation. For the sequencing of the inserted fragment, 20 μL of purified recombinant plasmid was sequenced by Nedayefan Co, Iran.
-
For optimization of protein production, various parameters were examined:supplementation of the medium at different temperatures (20, 30 and 37℃), induction with IPTG (isopropyl- -D-thiogalactoside) at concentrations ranging from 0.5 to 1 mmol/L. To find the optimal post induction time to harvest the cells, the progress of expression was followed by taking samples of the culture at different time points after induction (Table 1). The samples were analyzed directly by SDS-PAGE in order to achieve optimized conditions for expression with the pET-28a(+)-E7-stxB vector using E. coli BL21(DE3). This protocol included the following steps: Freshly transformed bacteria were grown overnight at 37℃ in 30 mL of 2X YT (Luria Broth (LB) medium with 2X yeast extract) medium containing kanamycin (40 μg/mL). The expression culture was inoculated with overnight culture in 1.5 L of 2X YT medium and grown at 37℃ with constant agitation at 220 rpm until the optical density at 600 nm reached 0.6 (OD600=0.6). The production of recombinant protein was induced by addition of IPTG to final concentrations of 0.5, 0.7 and 1 mmol/L. The cultures were allowed to grow for additional times of 3, 5 or 16 h and also at different temperatures (20, 30 and 37℃) (Table 1). The cells were harvested by centrifugation at 7085 rpm for 30 min and the cell pellet was stored at -20℃ until used for protein purification. Approximately 10 g (wet weight) of cells were obtained from 1.5 L of bacterial culture. Samples were subjected to SDS-PAGE and visualized by staining with Coomassie Brilliant Blue.
Condition label IPTG
(mmol/L)Time of Induction
(h)Temperature
(℃)Soluble recombinant protein yield* (%) 1 1 5 37 36 2 0.5 13 37 32 3 0.7 22 30 31 4 0.5 22 37 29 5 0.7 13 30 33 6 1 5 22 22 7 1 22 22 34 * The percentage of soluble recombinant protein yield of total cell protein. Table 1. Different induction conditions based on various parameters for optimization of protein production
-
Consistent with the previous protocol, the culture medium was supplemented with 10 μmol/L of ZnCl2, whereas the secretion of periplasmic proteins into the medium was stimulated by addition of 2% glycine and 1% Triton X-100 to the growth medium. This protocol included the following steps: Firstly, each supplemented medium was cultivated at 37℃ with constant agitation at 220 rpm until the optical density at 600 nm reached 0.6 (OD600=0.6). Then IPTG was added to final concentrations of 0.5, 0.7 and 1 mmol/L of the culture medium (Table 1). Afterwards, 2% glycine and 1% Triton X-100 were added to the culture medium when the optical density at 600 nm reached 4. The cultures were allowed to grow for additional times at the different temperatures listed above (Table 1). Finally, supernatant and cell debris were harvested by centrifugation at 7085 rpm for 30 min.
-
In each step, samples were analyzed by SDS–PAGE in a gel containing 12% (w/v) polyacrylamide under reducing conditions, followed by staining with Coomassie brilliant blue or Western blotting.
For Western blot analysis, non-stained SDS-PAGE gels were transferred to nitrocellulose membranes by electroblotting (Schleicher and Schuell BioScience, Dassel, Germany). The membranes were blocked for 1 h at room temperature in PBS (phosphate-buffered saline; 80 mmol/L Na2HPO4, 20 mmol/L KH2PO4, 137 mmol/L NaCl, 2. 7 mmol/L KCl, pH 7.4) containing 5% nonfat skim milk (Carl Roth, Karlsruhe, Germany). Then the membrane was incubated with rabbit anti His-tag polyclonal antibody against E7-STxB-His6 at 1:1000 dilution in PBS containing 0.1% Tween 20 (PBS-T), followed by a second incubation step with horseradish peroxidase-conjugated anti-mouse total IgG from rabbit (Sigma–Aldrich). Immunoreactivity was detected with diaminobenzidine (DAB) as a chromogenic substrate (Li Y L, et al., 2011).
-
Western blot analysis was performed for purified recombinant proteins. The intensity of Western blot was quantified by scanning densitometry (with TIFF or JPG formats) using the Image J analysis software package (NIH, USA).
-
For concentration of recombinant protein, the harvested supernatant was concentrated to 50 times by ultra filtration. The histidine-tagged E7-STxB protein was purified using a His-SelectTM Nickel Affinity Gel (3 mL; Sigma) following the manufacturer's standard procedures.
Enzymes, Vectors and Bacterial strains
Fusion steps and screening of the recombinant plasmids
Subcloning step for expression of the recombinant protein E7-Linker-STxB
Early optimization of the recombinant protein expression
Modification the culture medium using additives
SDS–PAGE and Western blotting
Quantification by densitometry
Concentration and Purification of recombinant E7-STxB-His6 protein secreted into supernatant
-
The produced construct, pET28a(+)-E7-STxB, was confirmed by 3 methods; First, by restriction enzyme analysis, then by PCR reaction of extracted vectors and finally by sequencing of the inserted fragment. All results confirmed an in-frame fusion of the construct. The PCR product of recombinant vector pET-28a(+)-E7-STxB was analyzed by agarose gel electrophoresis. BLAST searches of the fused protein sequence showed 100% identity at the amino acid sequence level with HPV16 E7 and shigella toxin B.
-
The predicted molecular weight of the expressed protein from sequence E7/STxB was 28 kDa. In preliminary experiments, the inductions were performed according to conditions shown in Table 1 (without additives). The pellet and supernatant fractions were tested. At first, via the SDS-PAGE analysis of early supernatant, we did not observe any secreted protein. After centrifugation of pellets which were resuspended in lysis buffer, secondary supernatants were reanalysed and the expected band around 28 kDa was observed. By comparing the gels, the best induction conditions to reach soluble protein were estimated to be as follows: 22 h after induction at 22℃, IPTG was added to a final concentration of 1 mmol/L and also at 5 h after induction at 37℃, with IPTG to a final concentration of 1 mmol/L (Fig. 1).
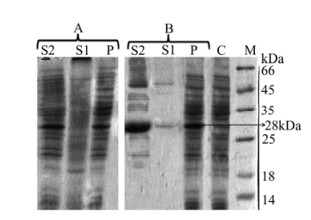
Figure 1. SDS-PAGE analysis of E7-STxB expression in E. coli BL21 (DE3) under different induction conditions A: 22 h after induction at 22℃. B: 5 h after induction at 37℃. C, soluble lysate of cells expressing recombinant plasmid before IPTG induction; P, pellet fractions of cells expressing recombinant plasmid after IPTG induction; S1, supernatant fractions of cells expressing recombinant plasmid after IPTG induction as secreted proteins; S2, soluble lysate of cells expressing recombinant plasmid after IPTG induction.
-
After addition of Triton X-100 and glycine to the culture medium at an OD600 of about 4, similar optimization conditions were observed for secretion of E7-STxB into the culture medium: 22 h after induction at 22℃ IPTG was added to a final concentration of 1 mmol/L. In contrast, under conditions of 5 h after induction at 37℃, very low level of secreted recombinant protein expression was observed (Fig. 2). Subsequent purification of secreted protein was straightforward. The E7/STxB protein, presented a molecular weight of approximately 28 kDa with only one band on SDS-PAGE.

Figure 2. SDS-PAGE analysis of E7-STxB expression in E. coli BL21 (DE3) under different induction conditions using additives; lane P, pellet fractions of cells expressing recombinant plasmid 22 h after IPTG induction at 22℃; lane A, supernatant fractions of cells expressing recombinant plasmid after 22 h induction at 22℃; lane B, supernatant fractions of cells expressing recombinant plasmid after 5 h induction at 37℃; lane M, protein molecular marker; lane C, soluble lysate of cells expressing recombinant plasmid before IPTG induction.
-
The purified proteins under optimized conditions were confirmed by Western blot assay. As shown in figure 3, fractions containing His-tagged protein are recognized by an antibody directed against E7-STxB-His6. The expressed protein appeared as the predominant band at 28 kDa.
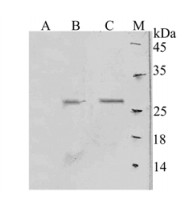
Figure 3. Western blot analysis of secreted E7-STxB-His6 into supernatant. M: protein molecular marker; A: Soluble lysate of cells without recombinant plasmid as negative control; B: 5 h induction at 37℃; C: 22 h induction at 22℃.
The concentration of purified soluble protein was quantified by subtracting normalized background values obtained from the pixel densities of bands corresponding to endogenous proteins. The intensity of optimized bands was measured to be > 6.2 times greater than other bands.
Construction of pET28a(+)-E7-STxB
The optimization of soluble protein expression
Secretory production of E7-STxB using additives
Western blotting and Densitometry of the E7/STxB fusion protein
-
This study describes a method for the optimization of soluble protein expression from a STxB-based vaccine in E. coli by applying protocol modifications in the form of media composition and culture conditions. The presence of inclusion bodies (IBs) in the periplasm is found to interfere with proper folding of the protein. Therefore, the secretion of protein into the supernatant may increase protein folding efficiency (Goncalves A N, et al., 2012). In this study, the ompA signal sequence has been used for translocation of recombinant protein to the periplasm for probable secretion into the growth medium. In spite of obtaining expressed E7-STxB that is soluble in a lysate of cells at the early optimization (Fig. 1), we did not observe secreted E7-STxB in the culture medium. In order to obtain a STxB-based vaccine with capability in tumor targeting (Haicheur N, et al., 2003; Janssen K P, et al., 2006; LaCasse E C, et al., 1996; Ohmura M, et al., 2005), devising a method to achieve a soluble form of the protein is necessary. We know that the host cell's capability to express the protein in a soluble fraction can be affected by a number of factors leading to the formation of soluble protein (Han L, et al., 2002; Sadraeian M, et al., 2011; Yang J, et al., 1998). These factors include:
-
Depending on whether the protein is eukaryotic or prokaryotic, the choice of a suitable host strain (herein E. coli BL21 (DE3)) as well as an appropriate signal sequence (ompA signal), can improve the chance of achieving a high yield of heterologous protein, provided that the recombinant protein is compatible with E. coli expression and folding mechanisms (Humphreys D P, et al., 2000; Ni Y, et al., 2009). In our approach, to further increase the expression level of the HPV16 E7 protein, we optimized the E7-coding sequence for the host strain.
-
According to previous reports, synergic addition of glycine and ZnCl2 is able to stabilize the membrane and prevent bacteriolysis. (Humphreys D P, et al., 2000). In this work, as Glycine and Triton X-100 were able to change morphology, causing the disruption of cell membrane integrity of E. coli, they were appropriate for releasing of recombinant proteins into the culture medium. Hence, by using lysis buffer, we confirmed the association effects of both substances on the solubility of E7-STxB.
-
Screening under the identified expression conditions caused a greater than six-fold improvement in the yield of soluble expression (Fig. 1). Subsequently, the supplemented media with additives (2% glycine and 1% Triton X-100) had greater than seven-fold improvement in the secreted production of E7-STxB (Fig. 2). We also showed that the expression at low temperatures (up to 22℃) for extended periods (up to 22 hours) led to improve the secretion of recombinant protein into the medium.
Use of genetically modified E. coli strains
Modification of media composition
Protein expression at lower temperature or in longer incubation time
-
This study demonstrates the importance of optimizing incubation parameters for recombinant protein expression in E. coli. It also shows that secretion production can be achieved over several hours when using additives such as glycine and Triton X-100. In fact, when the culture mediums were supplemented by additives, there was an inverse ratio between time of induction (TOI) and secreted protein level at lower temperatures (data not shown). The key to the success of this investigation was the ability to design a combined study in which we were able to co-modify both media composition and culture conditions. In this way, our study determined the optimal conditions for high yield soluble E7-STxB expression and subsequently facilitates achieving a functional soluble form of the protein for STxB-based vaccines.














 DownLoad:
DownLoad: