HTML
-
The actin cytoskeleton exists as a polarized array of filaments, termed F-actin, in dynamic equilibrium with a globular actin, or G-actin, pool. Polymerization of the F-actin filament occurs predominately at the barbed end (also known as the plus end), while depolymerization occurs predominantly at the pointed end (also known as the minus end). The precise spatiotemporal regulation of actin polymerization, mediated by actin-binding proteins (ABPs) and their upstream regulators, coordinates the force-generating and scaffolding properties of actin. These, in turn, regulate complex cellular processes including chemotaxis, cell adhesion, cytokinesis, the formation of cellular processes (microvilli, filopodia, lamellipodia, invadopodia, etc.), organelle movement, endoendocytosis, and the assembly of macromolecular domains during signal transduction and receptor internalization.
These primary functions of the actin cytoskeleton are themselves regulated by the actin-modulating ABPs, which exhibit various activities towards F-actin, including bundling and crosslinking (Fimbrin, Fascin, Filamin A, etc.), membrane and receptor association (ERM proteins, among others), capping (CapZ, tropomodulin, etc.), anti-capping (Ena/VASP proteins), nucleating and branching (the Arp2/3 complex), nucleation and polymerizing (Spire1/2 and formins), actin-associated motor activity (Myosins), and severing functions (cofilin, gelsolin, etc.). Directly upstream of these factors and their associated activities are the Rho-family monomeric GTPases, which are activated by nucleotide exchange of bound GDP to GTP. The Rho GTPases, which include 20 members, are divided into canonical signaling motifs that mediate particular cellular processes (Figure 1). Among these are the Rac1-PAK-LIMK-Cofilin pathway, which terminates with LIMK phosphorylating and inactivating cofilin; the Rac1-WAVE-Arp2/3 pathway, which promotes actin nucleation and branching, and lamellipodium generation; the Cdc42-WASP-Arp2/3 and Cdc42-mDia pathways, which activate Arp2/3 and the formin, mDia, respectively, and promote actin nucleation, branching, polymerization, and the formation of filopodia; and the RhoA-ROCK-LIMK-Cofilin and RhoA-ROCK-MLC pathways, which are partially responsible for the assembly of the contractile uropod, stress fibers, and focal adhesions.
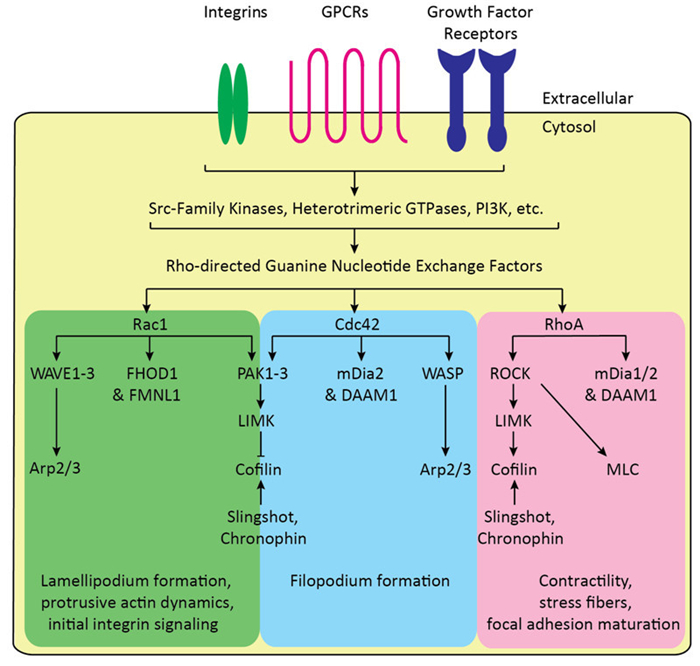
Figure 1. Canonical Rho GTPase signaling motifs regulate actin cytoskeletal dynamics. G Protein-Coupled Receptors (GPCRs), growth factor receptors, and integrin receptors directly activate upstream Src-family kinases and other non-receptor tyrosine kinases, PI3K-Akt signaling, other phosphoinositide kinase and phosphatases, Ras, and other signaling pathways that modulate Rho-directed guanine nucleotide exchange factors (GEFs). These, along with the GTPase-activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs) directly regulate Rho GTPases, with GEFs promoting GTPase activation, and GAPs and GDIs promoting inactivation and preventing activation, respectively. Rac1 directly activates the WAVE-Arp2/3 and PAK-LIMKCofilin, as well as activating the formins, FHOD1 and FMNL1: these pathways promote lamellipodium formation, initial adhesion signaling, and broadly protrusive actin dynamics. Similarly, Cdc42 activates the WASP/N-WASPArp2/3 and PAK-LIMK-Cofilin pathway, as well as activating the formins, mDia2 and DAAM1: these pathways also contribute to protrusive actin dynamics and filopodium formation. RhoA activates the ROCK-LIMK-Cofilin and ROCK-MLC pathway, as well as activating the formins, mDia1 and 2 and DAAM1: these pathways promote late integrin adhesion and maturation, as well as promoting stress fibers and contractility. Cofilin is also dynamically regulated by the phosphatases, Slingshot and Chronophin.
It is these force-generating and scaffolding properties exhibited by the actin cytoskeleton and its effectors that frequently become targets of necessity for viral replication, which also require these activities. As discussed in detail throughout this review, functions such as Arp2/3 nucleation and branching and formin-mediated actin polymerization are co-opted to produce force for viral motility. Similarly, the normal mechanisms of actin rearrangement, such as cofilin activation and inactivation cycling, are dysregulated to create favorable microcompartments for genome replication and nuclear localization. As such, each stage in the viral life cycle, from entry to egress and dissemination, will be considered in turn, explicating the various motifs of viral subversion of normal cellular actin signaling functions.
-
After engagement of the extracellular matrix (ECM) and the viral receptor, viruses must migrate to sites favorable for their particular mode of entry, whether it is direct plasma membrane fusion, or routes as divergent as macropinocytosis, phagocytosis, and the various forms of clathrin-mediated and clathrin-independent endocytosis. During this process, viruses frequently encounter actin-based processes, such as filopodia and microvilli, and utilize these structures to efficiently migrate to the cell membrane for entry.
Among the most common forms of this process is virus surfing, in which engagement of the receptor or ECM promotes movement of the attached virion towards the cell body in an actin-dependent mechanism. For instance, Lehmann et al. exhibited Murine leukemia virus (MLV), Avian leukosis virus (ALV), and Human immunodeficiency virus (HIV) surfing along filopodia in HEK293T cells transfected with their respective receptors (Lehmann M J, et al., 2005). MLV particles, in particular, migrated to the base of the filopodia and fused with the membrane, indicating that this was the active, and perhaps predominant, entry location (Lehmann M J, et al., 2005). Furthermore, this viral surfing was energy-dependent, being inhibited by sodium azide, and was dependent on functional myosin Ⅱ and F-actin: treatment of cells with the myosin Ⅱ inhibitor, blebbistatin, or cytochalasin D, which blocks F-actin barbed ends from polymerization, eliminated virus surfing and promoted random motility at the plasma membrane (Lehmann M J, et al., 2005). These treatments additionally inhibited MLV and ALV viral infection in rat XC cells, indicating that the viral surfing route contributed to productive infection (Lehmann M J, et al., 2005). The model developed around this process is centered around actin retrograde flow in which myosin Ⅱ generates forces that pull the filopodia-associated actin filaments towards the cell body, which is commensurate with viral receptor-actin association and filopodial tip actin polymerization. The net result is that the virus-receptor complex is processively pushed and dragged towards the cell body where entry occurs.
Viral surfing on filopodia has also been described for Herpes simplex virus type 1 (HSV-1) (Clement C, et al., 2006; Dixit R, et al., 2008), vaccinia virus (Mercer J, et al., 2008), Human papilloma virus 16 (HPV-16) (Schelhaas M, et al., 2008), Hepatitis C virus (HCV) (Coller K E, et al., 2009), and HIV-1 (Lehmann M J, et al., 2005), indicating that utilization of actin retrograde flow in cellular processes, particularly filopodia, may be a general mechanism for extracellular viral migration towards the cell body. To some extent, the intracellular migration of viruses is a virus-dependent active process, requiring signal transduction from or clustering of the viral receptor. For instance, HSV-1 not only migrated upon filopodia, but dramatically induced their formation in P19 neuron-like cells (Dixit R, et al., 2008). This indicates that HSV-1 engagement of the viral receptor specifically introduces signaling events to mediate filopodia formation, implicating that Rho and PI3K-dependent pathways are critical for mediating cytoskeletal structures essential for viral infection (Clement C, et al., 2006; Zheng K, et al., 2014b).
As indicated above for HSV-1, another common theme preceding cell entry is that virus-receptor engagements can actively signal to the actin cytoskeleton. This receptor-mediated signaling can dramatically affect entry events, promoting receptor clustering, receptor endocytosis, and fusion complex stabilization. Additionally, these signal transduction events often have important impacts on subsequent events in the viral life cycle. For instance, binding of HIV-1 to the chemokine coreceptor CXCR4/ CCR5 triggers the activation of Rac1-PAK1/2-LIMK-cofilin (Yoder A, et al., 2008; Vorster P J, et al., 2011). This signaling pathway regulates actin dynamics and the surface cycling of CXCR4, facilitating viral entry and post entry DNA synthesis and nuclear migration (Vorster P J, et al., 2011). Clustering of HIV-1 viral receptor and coreceptor (CD4 and either CCR5 or CXCR4) has also been suggested to be dependent on viral receptor signaling: specifically, engagement of both CD4 and CXCR4 activates Filamin A (Jiménez-Baranda S, et al., 2007), which promotes receptor motility and clustering. The ERM protein, Moesin, was also found to play a similar role in HIV-1 receptor clustering, in which the membrane receptor-actin crosslinking activity and receptor clustering occur after a viral receptor-mediated Moesin phosphorylation event (Barrero-Villar M, et al., 2009). It is probable that many other viruses utilize the actin cytoskeleton to induce receptor clustering and promote viral entry.
After engagement with the viral receptor, viral entry occurs either at the plasma membrane directly, or during/after internalization by one of the endocytic routes. Many, and perhaps all, of these routes depend on actin to one extent or another, particularly in primary, differentiated cells. For instance, macropinocytosis, which involves the Rac1-PAK-LIMK-Cofilin and, possibly, Rac1-WAVE-Arp2/3 pathways, has been shown to be required for HIV-1 entry into macrophages (Carter G C, et al., 2011). Macropinocytosis is also required for the entry of African swine fever virus (ASFV) (Sánchez E G, et al., 2012) and HPV-16 (Schelhaas M, et al., 2012). It also serves as an alternate entry route for Influenza A virus (IAV) (de Vries E, et al., 2011). An interesting twist on this is found in vaccinia virus entry, where viral envelope-associated phosphatidylserine induces an apoptotic body-mimicking signaling pathway, inducing a Rac1, PAK, tyrosine kinase, and actin-dependent macropinocytic entry route. Although, as reviewed by Moss (Moss B, 2012), the poxvirus entry fusion complex—composed of 11-12 glycosylated proteins—is abnormally complex, and other entry routes exist. Huttunem et al.(2014) also implicate a Rac1-dependent entry mechanism, involving neutral multivesicular bodies, in Coxsackievirus A9 entry (Huttunen M, et al., 2014). Similarly, HSV-1 has been shown to utilize a RhoA, tyrosine kinase, and actin-dependent phagocytosis-like route for entry (Clement C, et al., 2006; Zheng K, et al., 2014b). Poliovirus also utilizes an actin and tyrosine-kinase dependent route for entry, wherein genomic RNA release occurs in vesicles or membrane invaginations within 100-200 nm of the plasma membrane (Brandenburg B, et al., 2007). These examples, including many viruses from divergent groups, illuminate the need to co-opt the force generating and scaffolding functions of the cortical actin cytoskeleton, and the signaling pathways that modulate these functions, during viral entry.
-
After accessing the cortisol, most viruses must traverse the gap between the entry site and the nucleus for expression, genome replication, and, for some viruses, assembly. It is to this end that viruses utilize cellular force-generating apparatuses, which, along with dynein and kinesin for microtubule-based transport, include the motor and polymerizing proteins for the actin cytoskeleton; specifically, spire, formins, Arp2/3, and myosins. For instance, HIV-1 migrates to the nucleus utilizing two downstream components of Rac signaling, the Rac1-PAK1/2-LIMK-Cofiln pathway (Yoder A, et al., 2008; Vorster P J, et al., 2011), and the Rac1-WAVE2-Arp2/3 signaling pathway (Spear M, et al., 2014). The initial stimulus for Rac activation was also shown to require coreceptor—CCR5 or CXCR4—engagement and signal transduction (Yoder A, et al., 2008; Vorster PJ, et al., 2011; Spear M, et al., 2014). Interestingly, from a potential therapeutic standpoint, treatment of CD4 T lymphocytes with the Arp2/3 inhibitor, CK-548 (Nolen B J, et al., 2009), dramatically reduced HIV-1 replication at dosages that did not affect CD4 T cell activation or induce cytotoxicity (Spear M, et al., 2014). For viruses with larger genomes, more elaborate mechanisms for mediating nuclear localization exist. This is the case with baculovirus, where the p78/83 capsid encodes a WASP/ WAVE homology domain, the WASP homology-Cofilin homology/Connector-Acidic domain (WCA) (Machesky L M, et al., 2001). This domain is normally found in the Nuclear Promoting Factors (NPFs) of the WASP/WAVE family of proteins, whereupon NPF activation leads to WCA exposure and activation of the Arp2/3 complex for actin polymerization (Higgs H N, et al., 1999, 2001; Pollard T D, et al., 2003). However, in the presence of baculovirus p78/83, the viral WCA domain activates Arp2/3 at the viral core surface, generating forces and an actin comet tail that propel the core to the nucleus for expression and replication (Goley E D, et al., 2006; Ohkawa T, et al., 2010). As will be discussed later, nuclear translocation of the Arp2/3 complex, nuclear F-actin polymerization, and the resultant perturbation of nuclear structure also play a critical role during viral assembly and egress (Ohkawa T, et al., 1999; Goley E D, et al., 2006). HSV-1 has also been shown to utilize actin for intracellular motility and nuclear localization in a process involving the cofilin phosphatase and activator, slingshot, and calcium-regulated protease, calpain (Zheng K, et al., 2014a). The siRNA knockdown of slingshot and calpain reduced viral infection and led to a perinuclear localization of the viral cores, whereas wild-type virus localized to the nucleus efficaciously. In a somewhat stunning display of the requirements for specificity in actin signaling at distinct stages of viral replication, at later time points (8 hours post-infection and beyond), HSV-1 promotes ubiquitin/proteasome-dependent degradation of slingshot, leading to cofilin inactivation and promoting viral replication (Xiang Y, et al., 2014).
-
Once localized to the nucleus, or cytoplasm for large dsDNA viruses, viruses must concentrate, organize, and assemble their structural proteins, early-acting accessory and catalytic proteins, and genomic nucleic acids prior to egress of the viral particle. Needless to say, this requirement for colocalization of viral proteins, viral nucleic acids, and cellular factors requires scaffolding functions, and filamentous actin is often subverted for this purpose. This was shown for baculovirus: specifically, that mutation of the baculoviral CA domain dramatically abrogated nuclear F-actin accumulation and viral replication; furthermore, that viral progeny produced by one such mutant were distorted, either lacking an envelope altogether or having capsids that did not align with their envelope (Goley E D, et al., 2006).
In a similar fashion, Potato virus X (PVX), radically rearranges the ER and Golgi membranes and host actin into enormous assembly sites called the X-body (Tilsner J, et al., 2012). This process is mediated by the triple-gene block proteins (TGB1, 2, and 3), which are involved in cell-to-cell transfer through plasmodesmata, with PVX TGB1 being able to produce X-like-bodies when expressed alone(Tilsner J, et al., 2012). Although the role of actin in the X-body, and its relation to assembly was not specifically addressed, it has been shown that actin is required for intercellular transfer of PVX, in addition to Tobacco mosaic virus (TMV) and Tomato bushy stunt virus (TBSV), through plasmodesmata (Harries P A, et al., 2009). Thus, it is tempting to speculate that, even for certain plant viruses, actin may contribute to both assembly and cell-to-cell transfer.
Assembly of Influenza A virus (IAV) filamentous particles has also been shown to partially require an intact cortical actin cytoskeleton, as disruption with cytochalasin D (CCD) dramatically abrogated the titers of the filamentous, but not spherical, virions (Roberts P C, et al., 1998). This was later corroborated by Simpson-Holley et al.(2002), showing that treatment of MDCK cells with CCD, jasplakinolide (Jas), or latrunculin A (LatA) dramatically redistributed plasma membrane hemagglutinin (HA), lipid rafts, and actin into annular structures; there was also a commensurate loss of cell surface HAcontaining filamentous projections and filamentous virus production (Simpson-Holley M, et al., 2002a). The authors attributed the loss of filamentous viral assembly not to actin playing a specific role in assembly, per se, but rather mobilizing and recruiting lipid rafts to the assembly sites of filamentous particles, which, given their size, require a much larger pool of lipids than their spherical particle counterparts. As such, with lipid rafts, actin, and HA reorganized into small annuli, only spherical particle production occurs owing to the lack of sufficient lipid raft material for the larger filamentous particles (Simpson-Holley M, et al., 2002b).
Measles virus (MeV) has also been shown to utilize actin during assembly. For instance, disruption of the actin cytoskeleton rapidly reduced MeV particle release from the plasma membrane (Stallcup K C, et al., 1983). Furthermore, actin has been observed to colocalize with budding MeV, with meromyosin-labeled actin barbed ends protruding into the nascent virions in close juxtaposition with the viral nucleocapsid (Bohn W, et al., 1986). Additionally, the viral Matrix protein (M) was found to associate with F-actin, and this was shown to reduce the interaction with viral hemagglutinin (H) protein, as CCD treatment dramatically reduced actin co-immunoprecipitating with M, while enhancing H co-immunoprecipitation (Wakimoto H, et al., 2013). This study also indicated that there is a tradeoff between M-actin affinity and viral infectivity, wherein a higher affinity between M and H increases the amount of H, and hence the infectivity, of cell-free virus at the expense of cell-cell fusion. Furthermore, mutations in the M protein affecting M-actin affinity may arise in cell types where cellcell fusion is more productive than free viral infection (Wakimoto H, et al., 2013). In another study, Dietzel et al.(2013) exhibited that, while CCD treatment enhanced the rate of cell-cell fusion, it also reduces co-transport of M-RNP to the cell surface (Dietzel E, et al., 2013). Additionally, Jas treatment blocked free-virus production, while not affecting M-RNP co-transport, indicating that a dynamic actin cytoskeleton is required for cell-free virus maturation and release (Dietzel E, et al., 2013).
ROCK, LIMK, and cofilin have also been implicated in HIV-1 and Mason-Pfizer monkey virus assembly, release, and cell-cell transmission (Wen X, et al., 2014). Knockdown of LIMK did not inhibit HIV-1 maturation, yet, fully enveloped particles remained associated with the plasma membrane as mature virion aggregates (Wen X, et al., 2014). LIMK was also shown to be recruited to assembly sites (Wen X, et al., 2014). Although the exact mechanism for ROCK-LIMK-Cofilin contribution to virion release remains incompletely resolved, the authors suggested a factor retains the viral particles when normal cytoskeletal dynamics are disrupted (Wen X, et al., 2014).
-
Cell-to-cell transmission of animal viruses is a highly efficacious route of dissemination that minimizes interactions with the innate and adaptive immune system. Additionally, this process can render certain antivirals less effective, giving the issue increasing prominence in therapeutic research (Agosto L M, et al., 2014). It constitutes the infection of neighboring cells by an infected cell, typically mediated by the fusogenic surface protein or envelope glycoprotein.
Perhaps the best-studied example of this is Vaccinia virus cell-cell transmission (reviewed in (Welch M D, et al., 2013)). In 1976, Vaccinia virions were found to project from CCB-sensitive microvilli-like structures (Stokes G V, 1976); later, it was discovered that these virus-associated projections contained, in addition to actin, α-actinin (Hiller G, et al., 1979), fimbrin, and filamin (Hiller G, et al., 1981; Krempien U, et al., 1981). Additionally, Intracellular Enveloped Virions (IEVs) were shown to induce CCD-sensitive actin comet tails, reminiscent of Listeria, Shigella and Rickettsia infections (Cudmore S, et al., 1995). The actin comet-inducing factor, as identified by phenotypic characterization of deletion mutant viruses, was viral A36, a type 1b membrane protein (Röttger S, et al., 1999) that becomes tyrosine phosphorylated (Frischknecht F, et al., 1999a) at Y112 and Y132 (Frischknecht F, et al., 1999b). Y112 phosphorylation recruits the adaptor protein, Nck, via its SH2 domain (Frischknecht F, et al., 1999b), while Y132 was shown to recruit another adaptor protein, Grb2 (Scaplehorn N, et al., 2002). These proteins, in turn, help to recruit the N-WASP NPF complex (Frischknecht F, et al., 1999b; Moreau V, et al., 2000), which mediates Arp2/3 activation via its WCA domain. The result of this signaling cascade is the production of actin comet tails. When IEVs fuse with the plasma membrane, becoming Cellassociated Enveloped Virions (CEVs), viral A36 remains in the plasma membrane below the virion, promoting the production of the long, microvilli-like structures identified in 1976, and promoting viral dissemination (Welch M D, et al., 2013). Other factors that promote and regulate vaccinia virus motility are continually being discovered, including the formin, FHOD1, its upstream regulator, Rac1 (Alvarez D E, et al., 2013), and casein kinase 2 (Alvarez D E, et al., 2012).
HIV has also been shown to utilize an actin-dependent (Jolly C, et al., 2004, 2007) mode of transmission between T cells, in which viral Env glycoprotein on the donor cell organizes a polarized Virological Synapse (VS) that mediates highly efficient cell-cell transmission. This process may be up to 18, 000 times more efficacious that free viral infection (Chen P, et al., 2007). Morphologically, the VS and associated signaling complex, which contains the viral receptor, CD4, and viral coreceptor, CXCR4 or CCR5, resembles the Supramolecular Activation Complex (SMAC) observed in T cell activation (Vasiliver-Shamis G, et al., 2008), including the incorporation of the T cell Receptor (TCR) (Vasiliver-Shamis G, et al., 2009). Although the derived signals are insufficient to promote T cell activation, they do create an actin-depleted zone, which may facilitate viral infection at its earliest stages (Vasiliver-Shamis G, et al., 2009). Additionally, HIV has been shown to transfer between T cells using cellular nanotube processes, which may mimic aspects of the VS (Sowinski S, et al., 2008). An interesting twist on these forms of cell-to-cell transmission can be found in infected dendritic cells (DCs), wherein viral filopodia, containing immature virions at their tips, were observed (Aggarwal A, et al., 2012). These viral filopodia were found to be quite dynamic, allowing up to 800 CD4 T cell contacts per hour (Aggarwal A, et al., 2012). Additionally, these viral filopodia partially required the formin, Diaphanous-2 (Diaph2), and the viral Nef accessory protein (Aggarwal A, et al., 2012).
-
In addition to the more canonical modes of viral cooption of the actin cytoskeleton thus described, there is accumulating evidence that cytosolic and nuclear actin may play important roles in viral transcription, translation, and genome replication through somewhat unique modalities.
For instance, in HIV-1 infection, after entry into the host cell, the viral core is deposited on a dense meshwork of cortical actin that undergoes dynamics related to signal transduction mediated by the viral Env-CD4 and Env-CXCR4/CCR5 interactions (Jiménez-Baranda S, et al., 2007; Harmon B, et al., 2008, 2010; Yoder A, et al., 2008; Barrero-Villar M, et al., 2009; Vorster P J, et al., 2011; Spear M, et al., 2014). It is in this submembranous, dynamic actin cortex that the Reverse Transcriptase Complex (RTC) must convert the ssRNA genome into dsDNA. The first indication that the RTC may utilize the actin cytoskeleton came in 1995, when Hottiger et al. exhibited an interaction between beta-actin and the large subunit of Reverse Transcriptase (RT), or the Pol polyprotein precursor (Hottiger M, et al., 1995). Later, Bukrinskaya et al. (1998) corroborated these findings, showing that the RTC does indeed associate with the actin cytoskeleton in infected cells, and that pretreating cells with CCD, but not treatment 2 hours post-infection, reduced the accumulation of early and late reverse transcription products, with a more severe phenotype for late products (Bukrinskaya A, et al., 1998). These results indicated that actin disruption may directly impact the function of RT (Bukrinskaya A, et al., 1998). As was corroborated by later studies, CCD pretreatment reduced the nuclear accumulation of late RT products by 25-fold (Bukrinskaya A, et al., 1998). This process is presumably related to both the reliance on F-actin for both reverse transcription and nuclear migration of the Pre-Integration Complex (PIC) (Yoder A, et al., 2008; Cameron P U, et al., 2010; Vorster P J, et al., 2011; Spear M, et al., 2014). Although, the exact mechanism by which F-actin contributes to RTC activity remain unresolved. Nuclear actin bundles have also been implicated in late gene (gag) mRNA nuclear export and expression (Kimura T, et al., 2000): specifically, treatment with LatA, which disrupted viral Rev-RNP-induced nuclear actin bundles, caused retention of gag mRNA in the nucleus, decreasing cytosolic gag mRNA and, presumably, reducing Gag and GagPol protein expression (Kimura T, et al., 2000).
Rac1 has also been shown to be important for Influenza A virus (IAV) polymerase complex activity (Dierkes R, et al., 2014). Treatment of cells with a Rac1 inhibitor, NSC23766 (Gao Y, et al., 2004), reduced viral replication in A549 cells with an IC50 of 22 μmol/L (Dierkes R, et al., 2014). Furthermore, knockdown of TIAM1, the Rac1-activating GEF, or Rac1 itself also reduced viral replication (Dierkes R, et al., 2014). Additionally, NSC23766 reduced viral protein expression, which was later linked to inhibition of the viral polymerase complex (Dierkes R, et al., 2014). Fascinatingly, mice infected with IAV and treated with NSC23766 showed reduce titer of IAV in lung tissue, increased body weights, and a higher survivability than solvent-treated control mice (Dierkes R, et al., 2014). Although there was no specific study of whether NSC23766-mediated inhibition of the viral polymerase complex required actin, it is likely that actin played some role.
-
In this review, we have explored the multitude of mechanisms by which actin contributes to viral infection. Specially, we discussed how the scaffolding and force-generating properties of the actin cytoskeleton are subverted during almost every stage of viral replication. We also described how myosin Ⅱ-mediated actin retrograde flow is utilized by surfing viruses to access their entry site; how Arp2/3, formins, and Rho GTPase signaling are utilized to propel viral motility during nuclear migration and dissemination; how large actin molecular assemblies are co-opted for scaffolding functions during viral assembly, egress, and cell-to-cell transmission; and even some emerging, and poorly understood, functions of actin in viral genome replication events. Furthermore, it should be noted that, though outside the scope of this review, that actin modulators may represent unique targets for antiviral compound discovery.
-
This work is supported by US Public Health Service grant 1R01MH102144 from NIMH to Y. W.
-
All the authors declare that they have no competing interest. This article does not contain any studies with human or animal subjects performed by any of the authors.














 DownLoad:
DownLoad: