HTML
-
Hepatitis B virus belongs to hepadnaviridae, a family of enveloped viruses containing a partially double-stranded and circular DNA genome (GBirkenmeyer L, 2003). Records show that more than 350 million people worldwide are infected by this virus (Awan A R, et al., 2012). Clinical antiviral therapies currently used for chronic hepatitis B infection often lead to drug tolerance and are unable to eradicate the virus completely (Janssen H L, et al., 2005; Liaw Y F, et al., 2000; Niederau C, et al., 1996). Although an effective prophylactic vaccine has been available for nearly 30 years to date, up to 10% of individuals do not experience a response with an adequate antibody level to hepatitis B surface antigen (Sjogren M H, 2005). Therefore, development of a novel hepatitis B vaccine with a better penetrating and a higher response rate is essential.
The HBV envelope consists of three major immunogenic proteins, known as large (PreS1-PreS2-S), medium (PreS2-S) and small protein (HBsAg), and is the main component of the currently licensed recombinant prophylactic vaccine (Hadiji-Abbes N, et al., 2009). Previous studies have suggested that the PreS2-S region has the strongest immunogenicity, which could enhance antibody response to HBsAg and thus mitigate the chance of non-responsiveness of vaccines against escape variants, making it the most suitable candidate antigen for a novel HBV vaccine (Hadiji-Abbes N, et al., 2009). However, the entire PreS2 region containing 55 amino acids is sensitive to protease degradation, which makes large-scale preparation difficult (Langley K E, et al., 1988). The C-terminus of PreS2 containing 26 amino acids (120–145 aa) has been shown to be the most effective immu-nogen for production of antisera and has been proposed to contain a conserved conformational epitope critical for neutralization of infection (Chi S W, et al., 2006). Moreover, PreS2 containing 26 amino acids (120–145 aa) appears more stable than PreS2 containing 55 amino acids. Therefore, a truncated PreS2-S protein comprising 120–145 aa of the PreS2 (26 aa) C-terminus and entire HBsAg was used in the current study.
The methylotrophic yeast Hansenula polymorpha (H. polymorpha), a highly efficient microbial cell factory, has been successfully used in the production of several current commercial prophylactic HBV vaccines (Gellissen G, et al., 1992; Huang Y, et al., 2007). Here, H. polymorpha was employed as the host strain to express HBV PresS2-S, leading to a production yield of up to 250 mg per liter. After several steps of purification, total yield of ~15% with ~99% purity was obtained. Consistent with previous findings, ~22 nm virus-like particles were detected using electron microscopy.
-
H. polymorpha DL-1 (ATCC 26012) was purchased from ATCC, GeneticinTM (G418) from Invitrogen (Life Technologies, California, USA), the PCR cloning kit from Toyobo (Toyobo, Osaka, Japan) and CsCl from Sigma Aldrich (Sigma Aldrich, Missouri, USA). EcoR Ⅰ, Not Ⅰ, Bgl Ⅱ were purchased from Takara (Takara Bio Inc., Otsu, Japan).
-
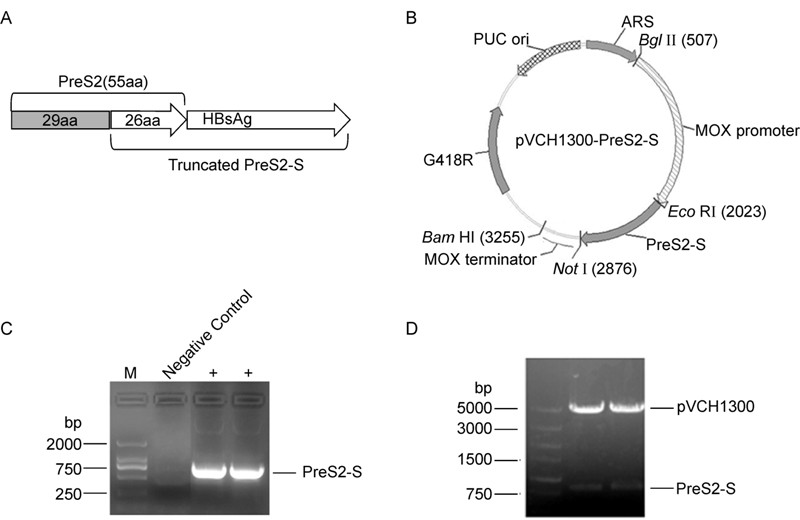
The codon-optimized coding sequence of adw 2-subtype HBV PreS2-S antigen (containing 26 aa of PreS2 C-terminus) was amplified from pVAC1010-PreS2-S (plasmid stored in our laboratory). Briefly, the PreS2-S was amplified by KOD plus kit according to the manufacturer'sinstructions using primer M26-F: CCGGAATTCGACATGGGTACAGTTAGTC (EcoR Ⅰ) and primer M-R: ATAGTTTAGCGGCCGCTTAAATGT (Not Ⅰ). The amplified coding sequence was subsequently digested with EcoR Ⅰ, Not Ⅰ and inserted into the expression vector, pVCH1300. The resultant plasmid was sequenced by GENEWIZ, Inc (Suzhou, China). For yeast transformation, plasmid p VCH1300-PreS2-S was linearized with the restriction endonuclease Bgl Ⅱ and transformed into H. polymorpha according to the method described by Faber et al. (1994). Positive clones were cultured in BMGY or YPD medium at 220 rpm/min, 37 ℃, for 1 day and induced using 1% (w/v) methanol for 48 h.
-
Harvested cells was re-suspended in sodium phosphate buffer (50 mmol/L PB, 150 mmol/L NaCl, pH 8.0) and disrupted with a high-pressure homogenizer at 4 ℃ (1500 bar, 4×). The cell debris was removed via centrifugation at 4 ℃, 4, 400g, for 15 min and the supernatant mixed with colloidal silica Aerosil-380 (Evonik Degussa, Essen, Germany), which was pre-equilibrated in 25 mmol/L sodium phosphate buffer, 0.5 mol/L NaCl, pH 7.2. The suspension was stirred at 4 ℃ for 4 h and centrifuged at 4 ℃, 7500 g, for 30 min. The pellet was washed with 25 mmol/L sodium phosphate buffer, pH 7.2, and centrifuged as above. The resultant pellet was re-suspended in 100 mmol/L sodium carbonate-bicarbonate buffer (pH 10.8) and incubated at 37 ℃ for 4 h. The suspension was centrifuged at 25 ℃, 10, 000 rpm, for 1 h. The clarified supernatant was adjusted to pH 8.0 and processed using ion-exchange chromatography (DEAE Sepharose FF, GE Healthcare). Bound PreS2-S was eluted using a salt step (50 mmol/L Tris-HCl buffer, pH 8.0, 0-1 mol/L NaCl). PreS2-S-containing fractions were pooled and further separated via CsCl density gradient centrifugation(Beckman, California, USA)at 36, 000 rpm and 4 ℃ for 16 h.
-
The PreS2-S concentration in the crude extract was determined using a commercially available ELISA kit (Kehua Bio-engineering, Shanghai, China). Purified PreS2-S was employed to establish a standard curve. Crude yeast extract containing PreS2-S was diluted appropriately with 0.1% BSA in PBS (pH 7.2) and applied for ELISA. The resultant absorption values were converted to PreS2-S concentrations using the standard curve established above. All samples were analyzed in triplicate.
-
The protein sample was separated using 12% SDSPAGE and characterized via silver staining ac cording to the manual of Novex (Life Technologies, California, USA). Western blot analysis was carried out using mouse anti-human HBsAg polyclonal antibody (ABMAX, Beijing, China) and HRP-conjugated goat anti-Mice IgG antibodies (Sigma Aldrich, Missouri, USA). Briefly, NC membrane was incubated with primary antibody (1:1000 dilution) in 5% blocking solution at 37 ℃ for 40 min. After washing with TBST, the membrane was incubated with HRP-conjugated secondary antibody (1:10000 dilution) in 5% blocking buffer at 37 ℃ for 30 min. Signal detection was performed using Western Lightning® Plus-ECL (PerkinElmer. Inc, Massachusetts, USA)
-
The structure of PreS 2-S virus-like particles was con-firmed using transmission electron microscopy (HT7700, Hitachi). Purified samples were applied to a 400-mesh copper grid, dried, and stained with 2% tungstophosphoric acid before TEM observation. The particle size was measured using Image J software.
Strains and reagents
Plasmid construction and protein expression
Protein purification
Quantitative measurement of PreS2-S using ELISA
Silver staining and Western Blot
Electron Microscopy
-
The coding sequence of the adw 2-subtype HBV PreS2-S antigen (containing 26 amino acids of the PreS2 C-terminus, 120–145 aa) was optimized according to the codon bias of H. polymorpha (Figure 1A). The optimized sequence was amplified and further cloned into the expression vector, pVCH1300, under the control of the strong methanol-inducing MOX promoter (Figure 1B and 1C). A geneticin (G418) resistance gene was used as a positive selection marker. The resultant plasmid was verified using colony PCR and restriction enzyme ana lysis (Figure 1D).
-
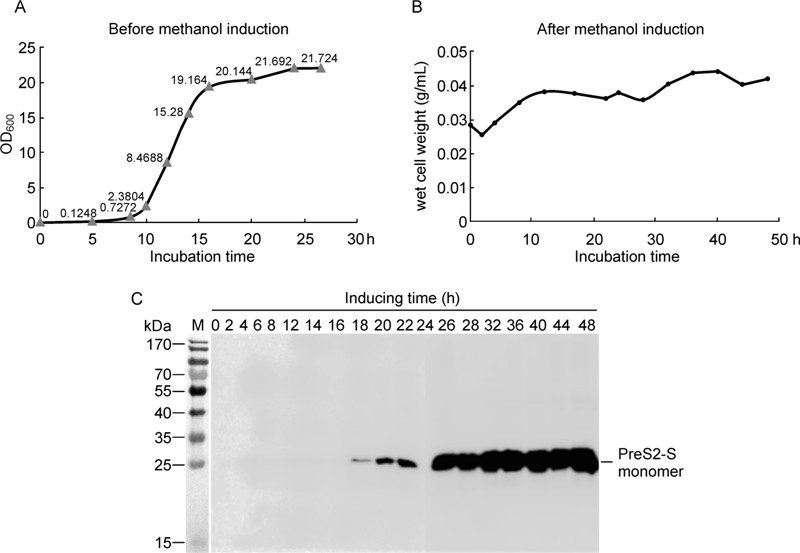
The plasmid was transformed into H. polymorpha cells using a previously published method (Faber K N, et al., 1994). Multiple copy inserts were screened according to protocols of the Invitrogen manual and further confirmed using rea1-time PCR (data not shown). The resultant multiple copy colonies were cultured in YPD medium and induced with methanol at a final concentration of 1% (Figure 2A and 2B). Induced samples were collected at the indicated time-points and characterized using Western blot (Figure 2C). After two days of methanol induction, the expression yield was estimated as 250 mg/L culture (2.5 mg/g wet cells).
-
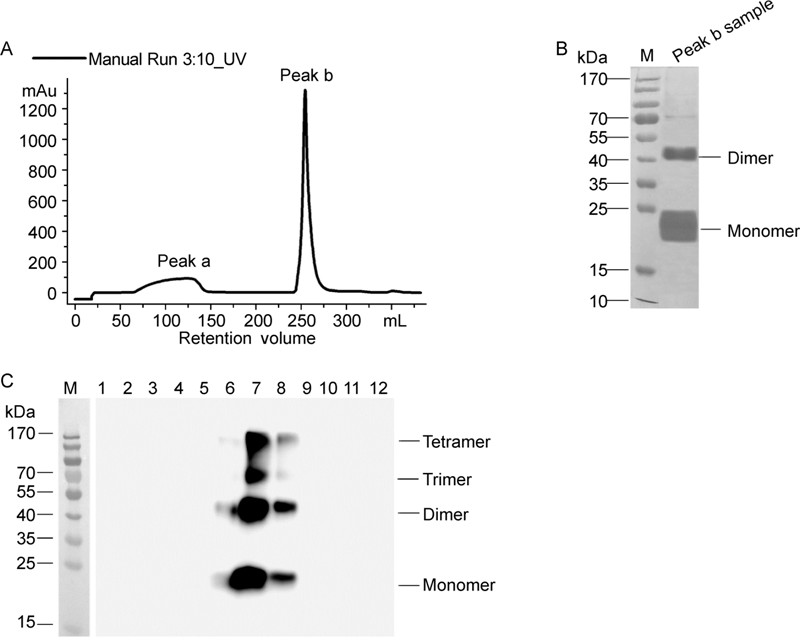
Harvested cells was re-suspended in sodium phosphate buffer and disrupted using a high-pressure homogenizer. After clarification via centrifugation at 4, 400 gfor 15 min, the supernatant containing HBsAg was further clarified with colloidal silica. The salt steps in the purification of PreS2-S protein using DEAE Sepharose FF chromatography are presented in Figure 3A. The SDS-PAGE pattern (Figure 3B) showed that the fraction eluted with buffer (50 mmol/L Tris-HCl pH 8.0 plus 500 mmol/L NaCl)mainly contained the monomer and polymer forms of the desired protein and no other evident bands of host protein. PreS2-S-containing fractions were pooled, fur ther separated via CsCl density gradient centrifugation, and analyzed via Western Blot. We observed enrichment of PreS2-S mainly in fractions 7–8 (~1.18 g/mL) (Figure 3C). The final product, with a total yield of ~15% and purity of 99%, was quantified using ELISA, as described above.
-
HBsAg needs to assemble into spherical particles, so-called virus-like particles (VLPs), to initiate the required protective immune response (Cabral G A, et al., 1978). To establish whether PreS2 is able to assemble into VLPs, transmission electron microscopy was performed. The hepatitis B PreS2-S antigen derived from H. polymorphacells organized into 22.6 ± 3.0 (n = 32) nm virus-like particles (Figure 4A and 4B), which were morphologically similar to those isolated from Saccharomyces cerevisiae and Pichia pastoris.
Plasmid construction
Expression of PreS2-S
Purification of HBV PreS2-S
Electron Microscopy findings
-
Hepatitis B virus (HBV) infection is the main cause of cirrhosis and hepatocellular cancer (Torbenson M, et al., 2002). The currently available recombinant prophylactic vaccine for HBV, either expressed in Chinese hamster ovary (CHO) cells or yeast, can induce effective humoral responses (Chen H, et al., 2012). However, up to 10% of individuals are still unable to experience a response with an adequate protective antibody level to hepatitis B surface antigen (Sjogren M H, 2005). Therefore, there remains an urgent requirement for a novel hepatitis B vaccine with a better penetrating and response rate.
The HBV envelope consists of three major immunogenic proteins, known as large (PreS1-PreS2-S), medium (PreS2-S) and small protein (HBsAg), respectively. The PreS2-S region has the strongest immunogenicity, which could accelerate antibody response to HBsAg and thus lower the chance of non-responsiveness of vaccines against escape variants (Hadiji-Abbes N, et al., 2009). Moreover, the PreS2 region contains a conserved polymerized albumin receptor site involved in virus attachment to human hepatocytes, and anti-PreS2 antibodies often arise earlier than anti-HBsAg antibodies (Kuroda S, et al., 1991; Milich D R, et al., 1985).
However, PreS2-S was comparatively unstable and easily degraded in our previous experiments (data no shown), consistent with other reports (Lian M, et al., 2008). Therefore, a truncated PreS2-S protein comprising the C-terminus of PreS2 (120–145 amino acids, 26 aa) and entire HBsAg was used in this study. Vaccines containing PreS2 can compete with HBV and block the route of infection, implying that PreS2 is a potential candidate component of novel HBV vaccines (Jiang Z G, et al., 2007).
The methylotrophic yeast, H. polymorpha, is a highly efficient microbial cell factory (Gellissen G, et al., 1992). Here, a PreS2 (26 aa)-S truncated gene was cloned into pVCH1300 and transformed into H. polymorpha DL-1. High dose G418-resistant colonies were screened and confirmed via real-time quantitative PCR. Remarkably, nearly 60 copies of plasmid were integrated into the genome of H. polymorpha DL-1 cells and transferred stably in progeny (data not shown), which possibly contributed to the significant production of foreign proteins. In our experiments, PreS2-S production yield in H. polymorpha cells was up to 250 mg per liter. Additionally, the generation process was more productive and cost-effective. After several steps of purification, a total yield of ~15% with ~99% purity was obtained, which was significantly higher than that fromPichia pastoris (Hardy E, et al., 2000). As a prerequisite for initiating the protective immune response, the ability to assemble into ~22 nm virus-like particles of PreS2-S antigen derived from H. polymorpha cells was confirmed using electron microscopy (Cabral G A, et al., 1978). To our knowledge, this is the first report of intracellular expression of PreS2-S VLPs in H. polymorpha. Further animal immunization studies with this antigen are required to fully evaluate PreS2-S as a potential vaccine candidate. Efficacy and safety studies will be reported in future publications.
-
We are grateful to our colleagues for sharing their results during scientific meetings and informal discussions. The Jiangsu Simcere Pharmaceutical Group has proprietary interests in the hepatitis B prophylactic and therapeutic vaccines described in this study.
-
All authors declare no competing interests. This article does not contain studies with human or animal subjects.
-
JL and HX designed the experiments. XX, SR, XC, JG, ZX, HH, HS, YG and TZ performed the experiments. XX, SR and JL analyzed data. XX and SR wrote the paper. All authors read and approved the final manuscript.















 DownLoad:
DownLoad: