HTML
-
Over the last several years, major efforts have been expended to study viral infection of honeybees mainly due to colony losses around the world (Allen M, et al., 1996). It seems that honeybees are infected with numerous viruses mounting to 18 so far. Infection may be asymptomatic but could still have adverse effects on the bee and may even cause death resulting in colony collapse. Sacbrood virus (SBV) is the most widely distributed of all honey bee viruses. Since its first identification in Apis mellifera L in the United State in 1913, infection of SBV has been found in almost all colonies throughout the world (Allen M, et al., 1996; Ellis J D, et al., 2005; Kim Cuc N T, et al., 2008; Ma M X, et al., 2010). SBV infects the brood and adults, but 2-day old larvae appear to be the most susceptible resulting in death (Ritter W, 1996). Diseased larvae do not pupate and ecdysial fluid collects around the integument in the form of a "sac", after which the disease has been designated. The color of infected larvae changes from pearly white to pale yellow. Following death, larvae dry out, making a dark brown gondola-shaped scale (Bailey L, 1975). SBV may infect adults without overt signs of disease (Anderson D L, et al., 1989; Bailey L, 1969). However, life span may become shorter (Bailey L, 1969; Wang D I, et al., 1970). Sacbrood occurs most frequently in the spring when the colony is in a very active growing phase (Bailey L, 1969).
The SBV was firstly described infecting the eastern honeybee, A. cerana, in Guangdong China in 1972 and was designated the Chinese sacbrood virus (CSBV). It emerged once again in 2008 in Liaoning, China (Ma M X, et al., 2010) causing death to individual bees or the collapse of the whole colony in an epidemic outbreaks. Later, the SBV was identified in A. cerana in Thailand and in Korea in 1982 and 2008, and named Thi SBV (TSBV) and AcSBV-Kor, respectively.
SBV is 26–30 nm in diameter, icosahedral, pseudo T = 3 structure symmetry, nonenveloped, and featureless in appearance with sedimentation coefficient of 160S and a buoyant density in cesium chloride of 1.33 g, and belongs to Picornaviridae. The outer shell of the capsid is composed of 60 repeated structures, each consisting of one molecule of 3 subunits VP1, VP2, and VP3 (25, 28, and 31.5 kDa), but each consisting of a single molecule of four subunits VP1, VP2, VP3, and VP4 (30.5, 31.5, 37.8 kDa and 44.2 kDa, respectively) about CSBV. The capsid proteins play important roles in the protection and in the determination of host specificity, tissue tropism and regional variations. Based on phylogeny and sequence homology of SBV-VP1, the SBV strains were divided into two major groups, an AC genotype infecting Apis cerana and an AM genotype infecting Apis mellifera, and also that the virus has host specificity and regional variations (Ma M X, et al., 2013).
-
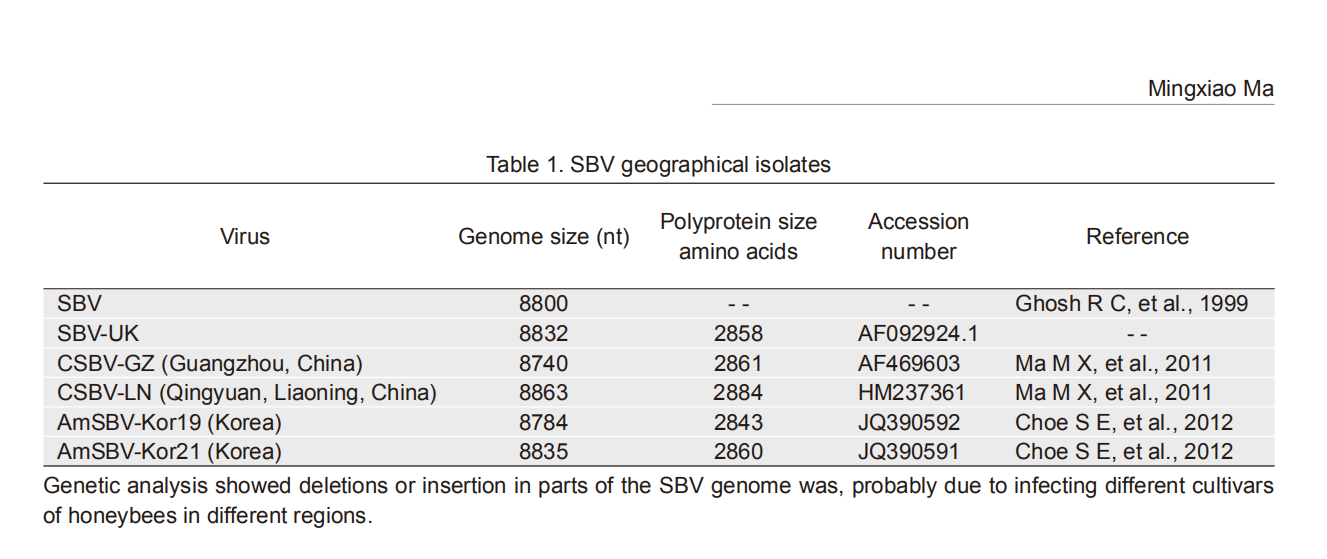
Among all the honeybee viruses, SBV RNA was the first to be totally sequenced. It has found that its genome is longer than that of typical mammalian picornaviruses of approximately 7 500 nt. The genome is a single stranded RNA with open reading frame that encodes a polyprotein. But the lengths of SBV genome are different because of origin from different strains as shown in Table 1. The Korean strains were isolated from Apis mellifera in 2011.
Virus Genome size (nt) Polyprotein size
amino acidsAccession
numberReference SBV 8800 -- -- Ghosh R C, et al., 1999 SBV-UK 8832 2858 AF092924.1 -- CSBV-GZ (Guangzhou, China) 8740 2861 AF469603 Ma M X, et al., 2011 CSBV-LN (Qingyuan, Liaoning, China) 8863 2884 HM237361 Ma M X, et al., 2011 AmSBV-Kor19 (Korea) 8784 2843 JQ390592 Choe S E, et al., 2012 AmSBV-Kor21 (Korea) 8835 2860 JQ390591 Choe S E, et al., 2012 Genetic analysis showed deletions or insertion in parts of the SBV genome was, probably due to infecting different cultivars of honeybees in different regions. Table 1. SBV geographical isolates
The genomic organization of SBV bears close resemblance to typical members of Picornaviridae, with the structural genes at the 5' end and the nonstructural genes at the 3' end and organized in a similar order. The RNA genome is surrounded by a capsid protein shell. The VPg (viral protein genome linked) is attached to the 5' end of the RNA genome via covalent bonds that confers stability to the 5' end and serves as a primer for replication and translation. A string of adenylic acid residue is linked to the 3' end of the genome, which can be folded into a stem-loop structure presumably involved in RNA replication.
-
SBV infects the brood and adult stages and is lethal to 2-day old larvae (Allen M, et al., 1996). The virus affects adult bees asymptomatically and infected adults may have a shorter life. In addition, the Apis cerana are more susceptible than Apis mellifera, which usually leads to colony collapse of the latter.
Food is a major conduit of virus transmission that thrives under the ideal conditions of a dense population density giving high rate of contact and high trophallactic rate. Evidence of the foodborne transmission pathway in bees has been provided by detection of SBV in food resources including pollen, royal jelly, honey, and in all developmental stages of bees, indicating that food in the colony is much involved in the dissemination of the virus and disease. Young larvae are infected by feeding on SBV-contaminated food, resulting in larval death. The nurse bees become infected while removing dead larvae or cleaning the infected bees' feces accumulated in the hive. Progeny virus amass in the hypopharyngeal glands of the nurse bees and these in turn disseminate it all through the colony by supplying their glandular secretion as food to larvae and also by exchanging food with other adult bees including foraging bees. In turn foraging bees disseminate SBV by passing it from their glandular secretions to collected pollen loads.
Infestation of bee colonies with varroa mites seems to have an association with SBV infection. It is generally agreed that varroa mites are the most important pest of honey bees. Varroa mites have evolved to spend their whole life cycle with the honey bee hosts, which feed on bees using their piercing mouthparts to penetrate the body walls and suck on the hemolymph. Mites can, therefore, act as vectors for pathogens during the feeding. Varroa mite-infested bee colonies are an abundant source of SBV (Antu'nez K, et al., 2006; Ball B V, et al., 1989; Bere'nyi O, et al., 2006). Detection of SBV in varroa mites (Chantawannakul P, et al., 2006; Shen M Q, et al., 2005; Tentcheva D, et al., 2004) indicates that the mites have the potential to transmit the virus in the colonies, although varroa mite as a vector in transmitting SBV has not yet been experimentally demonstrated.
Incidents of SBV in honey are notably seasonal. Rate of recurrence of SBV is significantly higher in spring and summer than in the fall (Anderson D L, et al., 1998; Bailey L, 1981; Tentcheva D, et al., 2004). The frequency of SBV infection in a honey bee colony appears to be directly related to the presence of susceptible brood and young workers. The abundance of nectar and pollen in spring and summer stimulate brood rearing resulting in the hatch of a large number of new workers, which provide healthy hosts to be attacked by SBV and proliferate in the colony. The variation in SBV incidence throughout the year is an indirect reflection of a variable susceptibility of the distinct bee developmental stages to the virus.
Disease by SBV occurs repeatedly, generally every 3–4 years. After 'self-cure', colonies infected during a reproduction period obtain a strong resistance to the virus. The colonies would then be screened out and reared as disease-resistant bee species. If the 'self-cure' occurred after reproduction period and the larval death witnessed a shard decrease, then it would be a false appearance. When reproduction is finished, the queen bee would lay fewer eggs or suspend spawning all together, causing a sharp decrease in larva numbers, and a minority of infected bees would emerge as a 'self-cure' phenomenon. Obviously, the 'selfcure' is mainly based on the reduction of infected subjects (namely, 2–3 days old larva), and would not genuinely reflect resistance to SBV. Pragmatically, the method of the prophylaxis and treatment of bee cystic larval disease adopted the false appearance of 'self-cure' and the characters of pathogen to control the full epidemic of bee cystic larva disease.
Many techniques have been utilized to detect viruses of bees viruses including enzyme-linked immunosorbent assay, immunodiffusion techniques (Allen M, et al., 1996), electron microscopy (EM), enhanced chemluminescent west ern blots (Allen M F, et al., 1986) and reverse transcriptase-PCR (RTPCR) (Benjeddou M, et al., 2001; Grabensteiner E, et al., 2000; Yan X, et al., 2009; Kukielka D, et al., 2009; Sanpa S, et al., 2009). The most widely used technique is immunodiffusion because it inexpensive, rapid, and quite specific (Allen M, et al., 1996) While serological methods are very useful, they are, however, limited to labs with facilities to produce abundant amounts of pure virus to raise antisera. EM investigations are done in major laboratories and are time consuming and relatively expensive for routine diagnosis. RT-PCR has been developed for honeybee viruses such as SBV (Grabensteiner E, et al., 2000; Kukielka D, et al., 2009; Sanpa S, et al., 2009), KBV (Sanpa S, et al., 2009; Evans J D, 2001), CSBV (Sanpa S, et al., 2009; Ma M X, et al., 2010) BQCV (Benjeddou M, et al., 2001; Sanpa S, et al., 2009) and ABPV (Benjeddou M, et al., 2001; Kukielka D, et al., 2009; Sanpa S, et al., 2009; Evans J D, 2001). Real-time RT-PCR has also been used to quantify virus preparations (Philippe B, et al., 2007; Yan X, et al., 2009). Loop-mediated isothermal amplification (LAMP) is a sensitive diagnostic method, relatively inexpensive and has been used to detect CSBV (Ma M X, et al., 2011). The methods of detecting SBV have been widely applied for different purpose.
-
In the prevention and treatment of SBV disease, close attention has to be paid to the comprehensive method of "Breeding disease-resistant bees" along with strengthening nutrition and management, proper drug therapy according to the feature of SBV and the intercurrence regularity of SBV disease. Drug therapy mainly adopted different Chinese herbal formulas, eg., formula 1: 50 g lithospermum, 30 g honeysuckle, 5 g liquorice; formula 2: 40 g cortex acanthopanacis, 20 g honeysuckle, 10 g cassia twig, 25 g root of rehmannia; formula 3: 30 g Cyrtomium fortunei, 30 g honeysuckle, 5 g liquorice. Basically, any of the formulae is first boiled in a specific volume of the water to extract the active ingredients from the herbs, filtered and concentrated to about 250 mL volume. The extract is then mixed in a 1:1 ration with soap and honey.. Treatment should be applied on alternate days for 4–5 consecutive times. Each dose is sufficient to treat 10–15 cases (hives). This traditional Chinese medicine treatment may give out a pungent smell and may cause bees fly away. Our laboratory has recently developed an SBV egg yolk antibody and was used to treat SBV-infected bees with positive effect. A clinical validation test is undergoing.
-
In recent years, progress in SBV research has been impressive. However, the full picture of infection of honeybees by SBV is yet to be realized particularly at the molecular level. Indeed, many gaps in our understanding of fundamental processes underlying the dynamics of virus transmission, pathogenesis, viral epidemics and immunity of the host. For example, what mechanisms regulate virus transmission, or what is the role of viral gene expression in pathogenesis, and how do the host immune responses affect virus survival and multiplication? One of the bottle necks in investigating SBV has been the lack of a permissive cell culture system. A permissive cell cultures would provide investigators with a valuable tool elucidate the full cycle of virus infection in cells and to address intriguing questions on virus/host interactions. A permissive cell culture would solve this deadlock that has hampered efforts to isolate and elucidate the pathogens. A permissive cell culture would also facilitate a controlled system to study the fundamental processes of virus infection and could provide means to propagate and generate highly purifies virus and may also help in studies on virus pathology.
Genome organization and classification
Epidemiology
Prevention and treatment
Future directions
-
This work was supported by grants from the National Natural Science Foundation of China (No. 31372435). All the authors declare that they have no competing interest. This article does not contain any studies with human participants or animals performed by any of the authors.














 DownLoad:
DownLoad: